| PILRA | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | PILRA, FDF03, paired immunoglobin like type 2 receptor alpha | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 605341 MGI: 2450529 HomoloGene: 8387 GeneCards: PILRA | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Paired immunoglobin like type 2 receptor alpha is a protein that in humans is encoded by the PILRA gene. [5]
Function
Cell signaling pathways rely on a dynamic interaction between activating and inhibiting processes. SHP-1-mediated dephosphorylation of protein tyrosine residues is central to the regulation of several cell signaling pathways. Two types of inhibitory receptor superfamily members are immunoreceptor tyrosine-based inhibitory motif (ITIM)-bearing receptors and their non-ITIM-bearing, activating counterparts.
Control of cell signaling via SHP-1 is thought to occur through a balance between PILRalpha-mediated inhibition and PILRbeta-mediated activation. These paired immunoglobulin-like receptor genes are located in a tandem head-to-tail orientation on chromosome 7. This particular gene encodes the ITIM-bearing member of the receptor pair, which functions in the inhibitory role. Alternative splicing has been observed at this locus, and three variants, each encoding a distinct isoform, are described.
In contrast to PILRbeta, which has only one known natural ligand, PILRalpha has many known protein-protein interactions.[6] PILRalpha recruits PTPN6 and PTPN1 via interactions of its ITIM motifs.[7] PILRalpha is also used by some viruses, notably HSV-1, for cell entry.[6][8]
Structure
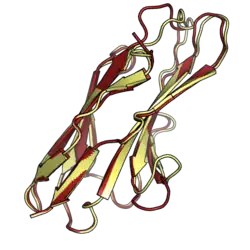
As with other paired receptors, PILRalpha has a longer cytoplasmic tail compared to PILRbeta and features two intracellular ITIM motifs.[7][9] PILRalpha has an extracellular domain with a siglec-like immunoglobulin fold that substitutes hydrophobic interactions for the siglec fold's characteristic disulfide bond. The structure of this domain is very similar to that of PILRbeta, but the two proteins nevertheless have different binding affinities for sialic acid.[6]
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000085514 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000046245 - Ensembl, May 2017
- ↑ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ "Entrez Gene: Paired immunoglobin like type 2 receptor alpha". Retrieved 2017-01-10.
- 1 2 3 Lu Q, Lu G, Qi J, Wang H, Xuan Y, Wang Q, et al. (June 2014). "PILRα and PILRβ have a siglec fold and provide the basis of binding to sialic acid". Proceedings of the National Academy of Sciences of the United States of America. 111 (22): 8221–6. Bibcode:2014PNAS..111.8221L. doi:10.1073/pnas.1320716111. PMC 4050567. PMID 24843130.
- 1 2 Mousseau DD, Banville D, L'Abbé D, Bouchard P, Shen SH (February 2000). "PILRalpha, a novel immunoreceptor tyrosine-based inhibitory motif-bearing protein, recruits SHP-1 upon tyrosine phosphorylation and is paired with the truncated counterpart PILRbeta". The Journal of Biological Chemistry. 275 (6): 4467–74. doi:10.1074/jbc.275.6.4467. PMID 10660620.
- ↑ Furukawa A, Kakita K, Yamada T, Ishizuka M, Sakamoto J, Hatori N, et al. (December 2017). "Structural and thermodynamic analyses reveal critical features of glycopeptide recognition by the human PILRα immune cell receptor". The Journal of Biological Chemistry. 292 (51): 21128–21136. doi:10.1074/jbc.M117.799239. PMC 5743085. PMID 29046357.
- ↑ Wilson MD, Cheung J, Martindale DW, Scherer SW, Koop BF (November 2006). "Comparative analysis of the paired immunoglobulin-like receptor (PILR) locus in six mammalian genomes: duplication, conversion, and the birth of new genes". Physiological Genomics. 27 (3): 201–18. doi:10.1152/physiolgenomics.00284.2005. PMID 16926269.
Further reading
- Tabata S, Kuroki K, Maita N, Wang J, Shiratori I, Arase H, et al. (January 2008). "Expression, crystallization and preliminary X-ray diffraction analysis of human paired Ig-like type 2 receptor alpha (PILRalpha)". Acta Crystallographica. Section F, Structural Biology and Crystallization Communications. 64 (Pt 1): 44–6. doi:10.1107/S1744309107065384. PMC 2373998. PMID 18097101.
- Satoh T, Arii J, Suenaga T, Wang J, Kogure A, Uehori J, et al. (March 2008). "PILRalpha is a herpes simplex virus-1 entry coreceptor that associates with glycoprotein B". Cell. 132 (6): 935–44. doi:10.1016/j.cell.2008.01.043. PMC 2394663. PMID 18358807.
- Arii J, Uema M, Morimoto T, Sagara H, Akashi H, Ono E, et al. (May 2009). "Entry of herpes simplex virus 1 and other alphaherpesviruses via the paired immunoglobulin-like type 2 receptor alpha". Journal of Virology. 83 (9): 4520–7. doi:10.1128/JVI.02601-08. PMC 2668467. PMID 19244335.
- Fan Q, Lin E, Satoh T, Arase H, Spear PG (August 2009). "Differential effects on cell fusion activity of mutations in herpes simplex virus 1 glycoprotein B (gB) dependent on whether a gD receptor or a gB receptor is overexpressed". Journal of Virology. 83 (15): 7384–90. doi:10.1128/JVI.00087-09. PMC 2708615. PMID 19457990.
- Kogure A, Shiratori I, Wang J, Lanier LL, Arase H (February 2011). "PANP is a novel O-glycosylated PILRα ligand expressed in neural tissues". Biochemical and Biophysical Research Communications. 405 (3): 428–33. doi:10.1016/j.bbrc.2011.01.047. PMC 4089865. PMID 21241660.
- Sun Y, Senger K, Baginski TK, Mazloom A, Chinn Y, Pantua H, et al. (May 2012). "Evolutionarily conserved paired immunoglobulin-like receptor α (PILRα) domain mediates its interaction with diverse sialylated ligands". The Journal of Biological Chemistry. 287 (19): 15837–50. doi:10.1074/jbc.M111.286633. PMC 3346071. PMID 22396535.
- Chowdhury S, Naderi M, Chouljenko VN, Walker JD, Kousoulas KG (June 2012). "Amino acid differences in glycoproteins B (gB), C (gC), H (gH) and L (gL) are associated with enhanced herpes simplex virus type-1 (McKrae) entry via the paired immunoglobulin-like type-2 receptor α". Virology Journal. 9: 112. doi:10.1186/1743-422X-9-112. PMC 3402990. PMID 22695228.
- Chowdhury S, Chouljenko VN, Naderi M, Kousoulas KG (March 2013). "The amino terminus of herpes simplex virus 1 glycoprotein K is required for virion entry via the paired immunoglobulin-like type-2 receptor alpha". Journal of Virology. 87 (6): 3305–13. doi:10.1128/JVI.02982-12. PMC 3592154. PMID 23302878.
- Lun YZ, Chi Q, Wang XL, Wang F, Sui W (February 2014). "Identification of paired immunoglobulin-like type 2 receptor α as hepatitis B virus DNA polymerase transactivated protein 1 interacting proteins". Molecular Medicine Reports. 9 (2): 720–4. doi:10.3892/mmr.2013.1813. PMID 24253495.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.