 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
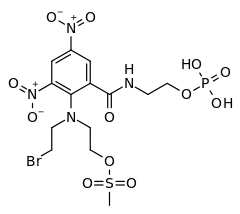
| Formula | C14H20BrN4O12PS |
| Molar mass | 579.27 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
PR-104 is a drug from the class of hypoxia-activated prodrugs (HAPs),[1] which is being researched as a potential anti-cancer therapeutic agent. It is a phosphate ester “pre-prodrug” that is rapidly converted to the HAP PR-104A in the body. PR-104A is in turn metabolised to reactive nitrogen mustard DNA crosslinking agents in hypoxic tissues such as found in solid tumours.[2][3][4] Following initial clinical studies,[5][6] it was discovered that PR-104A is also activated by the enzyme AKR1C3, independently of hypoxia.[7] Hypoxia in the bone marrow of patients with leukaemia,[8][9] and high activity of AKR1C3 in some leukaemia subtypes [10][11][12] has led to interest in clinical trials of PR-104 in relapsed refractory acute leukaemias.[13][14]
References
- ↑ Wilson WR, Hay MP (June 2011). "Targeting hypoxia in cancer therapy". Nature Reviews. Cancer. 11 (6): 393–410. doi:10.1038/nrc3064. PMID 21606941. S2CID 36040922.
- ↑ Patterson AV, Ferry DM, Edmunds SJ, Gu Y, Singleton RS, Patel K, et al. (July 2007). "Mechanism of action and preclinical antitumor activity of the novel hypoxia-activated DNA cross-linking agent PR-104". Clinical Cancer Research. 13 (13): 3922–32. doi:10.1158/1078-0432.CCR-07-0478. PMID 17606726.
- ↑ Singleton RS, Guise CP, Ferry DM, Pullen SM, Dorie MJ, Brown JM, et al. (May 2009). "DNA cross-links in human tumor cells exposed to the prodrug PR-104A: relationships to hypoxia, bioreductive metabolism, and cytotoxicity". Cancer Research. 69 (9): 3884–91. doi:10.1158/0008-5472.CAN-08-4023. PMID 19366798.
- ↑ Foehrenbacher A, Secomb TW, Wilson WR, Hicks KO (December 2013). "Design of optimized hypoxia-activated prodrugs using pharmacokinetic/pharmacodynamic modeling". Frontiers in Oncology. 3: 314. doi:10.3389/fonc.2013.00314. PMC 3873531. PMID 24409417.
- ↑ Jameson MB, Rischin D, Pegram M, Gutheil J, Patterson AV, Denny WA, Wilson WR (March 2010). "A phase I trial of PR-104, a nitrogen mustard prodrug activated by both hypoxia and aldo-keto reductase 1C3, in patients with solid tumors". Cancer Chemotherapy and Pharmacology. 65 (4): 791–801. doi:10.1007/s00280-009-1188-1. PMID 20012293. S2CID 35209191.
- ↑ McKeage MJ, Gu Y, Wilson WR, Hill A, Amies K, Melink TJ, Jameson MB (October 2011). "A phase I trial of PR-104, a pre-prodrug of the bioreductive prodrug PR-104A, given weekly to solid tumour patients". BMC Cancer. 11 (1): 432. doi:10.1186/1471-2407-11-432. PMC 3205073. PMID 21982454.
- ↑ Guise CP, Abbattista MR, Singleton RS, Holford SD, Connolly J, Dachs GU, et al. (February 2010). "The bioreductive prodrug PR-104A is activated under aerobic conditions by human aldo-keto reductase 1C3". Cancer Research. 70 (4): 1573–84. doi:10.1158/0008-5472.CAN-09-3237. PMID 20145130.
- ↑ Benito J, Shi Y, Szymanska B, Carol H, Boehm I, Lu H, et al. (11 August 2011). "Pronounced hypoxia in models of murine and human leukemia: high efficacy of hypoxia-activated prodrug PR-104". PLOS ONE. 6 (8): e23108. Bibcode:2011PLoSO...623108B. doi:10.1371/journal.pone.0023108. PMC 3154919. PMID 21853076.
- ↑ Benito J, Zeng Z, Konopleva M, Wilson WR (August 2013). "Targeting hypoxia in the leukemia microenvironment". International Journal of Hematologic Oncology. 2 (4): 279–288. doi:10.2217/ijh.13.32. PMC 3905090. PMID 24490034.
- ↑ Jamieson SM, Gu Y, Manesh DM, El-Hoss J, Jing D, Mackenzie KL, et al. (March 2014). "A novel fluorometric assay for aldo-keto reductase 1C3 predicts metabolic activation of the nitrogen mustard prodrug PR-104A in human leukaemia cells". Biochemical Pharmacology. 88 (1): 36–45. doi:10.1016/j.bcp.2013.12.019. PMID 24434189.
- ↑ Moradi Manesh D, El-Hoss J, Evans K, Richmond J, Toscan CE, Bracken LS, et al. (September 2015). "AKR1C3 is a biomarker of sensitivity to PR-104 in preclinical models of T-cell acute lymphoblastic leukemia". Blood. 126 (10): 1193–202. doi:10.1182/blood-2014-12-618900. PMC 4559932. PMID 26116659.
- ↑ Abbattista MR, Ashoorzadeh A, Guise CP, Mowday AM, Mittra R, Silva S, et al. (November 2021). "Restoring Tumour Selectivity of the Bioreductive Prodrug PR-104 by Developing an Analogue Resistant to Aerobic Metabolism by Human Aldo-Keto Reductase 1C3". Pharmaceuticals. 14 (12): 1231. doi:10.3390/ph14121231. PMC 8707548. PMID 34959631.
- ↑ Konopleva M, Thall PF, Yi CA, Borthakur G, Coveler A, Bueso-Ramos C, et al. (July 2015). "Phase I/II study of the hypoxia-activated prodrug PR104 in refractory/relapsed acute myeloid leukemia and acute lymphoblastic leukemia". Haematologica. 100 (7): 927–34. doi:10.3324/haematol.2014.118455. PMC 4486227. PMID 25682597.
- ↑ Phillips RM (March 2016). "Targeting the hypoxic fraction of tumours using hypoxia-activated prodrugs". Cancer Chemotherapy and Pharmacology. 77 (3): 441–57. doi:10.1007/s00280-015-2920-7. PMC 4767869. PMID 26811177.