 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral[1] |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
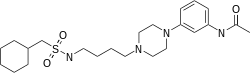
| Formula | C23H38N4O3S |
| Molar mass | 450.64 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Naluzotan (INN, USAN; PRX-00023) is a serotonergic drug of the phenylpiperazine class that was under investigation by EPIX Pharmaceuticals Inc for the treatment of generalized anxiety disorder and major depressive disorder.[1][2] It acts as a selective and potent 5-HT1A receptor partial agonist,[2][3] readily stimulating prolactin responses,[4] though it has also been found to bind to and activate the σ receptor.[5] Naluzotan was well tolerated in clinical trials,[4] with more patients in the control group dropping out due to adverse effects than in the active group in one study.[2] The most frequently reported side effect was headache in 15% of patients (compared to 10% for placebo).[2] In addition, naluzotan demonstrated significant antidepressant and anxiolytic effects as per the HAM-D and MADRS and the HAM-A, respectively, in some trials,[2] but in others it did not.[6][7] In the end it was not found to be significantly superior enough to placebo and development was stopped.[7]
See also
References
- 1 2 de Paulis T (2007). "Drug evaluation: PRX-00023, a selective 5-HT1A receptor agonist for depression". Curr Opin Investig Drugs. 8 (1): 78–86. PMID 17263189.
- 1 2 3 4 5 Rickels K, Mathew S, Banov MD, Zimbroff DL, Oshana S, Parsons EC, Donahue SR, Kauffman M, Iyer GR, Reinhard JF (2008). "Effects of PRX-00023, a novel, selective serotonin 1A receptor agonist on measures of anxiety and depression in generalized anxiety disorder: results of a double-blind, placebo-controlled trial". J Clin Psychopharmacol. 28 (2): 235–239. doi:10.1097/JCP.0b013e31816774de. PMID 18344738. S2CID 40515142.
- ↑ Becker OM, Dhanoa DS, Marantz Y, Chen D, Shacham S, Cheruku S, Heifetz A, Mohanty P, Fichman M, Sharadendu A, Nudelman R, Kauffman M, Noiman S (2006). "An integrated in silico 3D model-driven discovery of a novel, potent, and selective amidosulfonamide 5-HT1A agonist (PRX-00023) for the treatment of anxiety and depression". J Med Chem. 49 (11): 3116–3135. doi:10.1021/jm0508641. PMID 16722631.
- 1 2 Iyer GR, Reinhard JF, Oshana S, Kauffman M, Donahue S (2007). "Tolerability, pharmacokinetics, and neuroendocrine effects of PRX-00023, a novel 5-HT1A agonist, in healthy subjects". J Clin Pharmacol. 47 (7): 817–824. doi:10.1177/0091270007300953. PMID 17495280. S2CID 30536648.
- ↑ Prof John Kelly (2010). Principles of CNS Drug Development: From Test Tube to Patient. New York: Wiley. ISBN 978-0-470-51979-0.
- ↑ Mathew SJ, Garakani A, Reinhard JF, Oshana S, Donahue S (2008). "Short-term tolerability of a nonazapirone selective serotonin 1A agonist in adults with generalized anxiety disorder: a 28-day, open-label study". Clin. Ther. 30 (9): 1658–1666. doi:10.1016/j.clinthera.2008.09.006. PMID 18840371.
- 1 2 Kirchhoff VD, Nguyen HT, Soczynska JK, Woldeyohannes H, McIntyre RS (October 2009). "Discontinued psychiatric drugs in 2008". Expert Opinion on Investigational Drugs. 18 (10): 1431–43. doi:10.1517/13543780903184591. PMID 19715445. S2CID 34201544.