Palladacycle, as a class of metallacycles, refers to complexes containing at least one carbon-palladium bond. Palladacycles are invoked as intermediates in catalytic or palladium mediated reactions. They have been investigated as pre-catalysts for homogeneous catalysis and synthesis.
History of the palladacycle discovery
In the 1960s, Arthur C. Cope and Robert W. Siekman reported the cyclopalladation reaction between aromatic azobenzenes and palladium(II) dichloride.[1] The potential of palladacycles as catalysts was highlighted by Herrmann's catalyst in 1990s. Derivatives of tris(o-tolyl)phosphine proved effective in Heck reactions.[2]

Classes of palladacycles
There are two distinct types of palladacycle: four-electron donor (CY) and six-electron donor (YCY) complexes.

Neutral, cationic and anionic palladacycles
The palladacycles can be neutral, cationic, or anionic. Depending on the nature of the coordinating ligands, the neutral palladacycles can be monomers, dimers, or bis-cyclopalladated.

Palladacycles with various ring-sizes
Palladacycles with ring-sizes range from 3 to 10 have been synthesized and characterized, whereas only 5-/6-membered ones are commonly used. Palladacycles of 3-/4-/>6-membered ring-sizes are usually unstable due to their ring strains.

Palladacycles with various donor groups
The palladacycles could also be classified by the donor atoms. For example, the Herrmann’s catalyst discussed before is a phosphine-derived palladacycle. Other types of palladacycles such as phosphite palladacycle, imine palladacycle, oxime palladacycle, CS-/CO-palladacycles are also effective in catalytic reactions. Palladacycles derived from 2-aminobiphenyl have been used in a variety of cross-coupling reactions.
Synthesis of palladacycles
Several methods are available for the preparation of palladacycles. A simple and direct method is C–H activation.[3] The cyclopalladation of aromatic derivatives is usually considered to go through an electrophilic aromatic substitution pathway.[4] The oxidative addition of aryl halides is another useful method.[5] However, the accessibility of the aryl halides starting material is a major drawback.

Other types of reactions such as transmetalation[6] and nucleopalladation[7] also turned out to be effective methods in the synthesis of palladacycles.
Applications as precatalysts
Palladacycles are used as pre-catalysts, usually by the reductive elimination from palladium(II) to the catalytically active palladium(0). In the example of 2-aminobiphenyl palladacycles, a kinetically active 12-electrons Pd(0) species is formed, allowing for further oxidative addition with reactants.[8] A series of 2-aminobiphenyl bearing various X and L groups were synthesized to better understand the electron/steric effect.
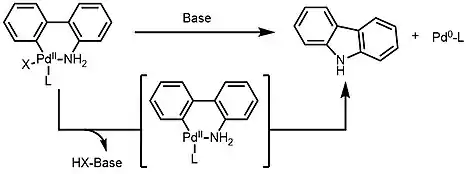
By employing palladacycles as pre-catalysts, high reactivity and selectivity have been achieved in Heck reaction[2] and a variety of cross-coupling reactions, such as Suzuki,[9] Sonogashira,[10] Stille,[11] Buchwald–Hartwig reactions.[12]
Total synthesis containing palladacycles have been demonstrated.[13][14]

Other applications
Except their abilities in catalyzing organic reactions, palladacycles have also shown their potential in medicinal and biological chemistry after the success of cis-Pt(NH3)2Cl2 as an anticancer agent. Additionally, they can also be used in CO/SCN- sensing.[15]
Further reading
- Beletskaya, Irina P.; Cheprakov, Andrei V. (November 2004). "Palladacycles in catalysis – a critical survey". Journal of Organometallic Chemistry. 689 (24): 4055–4082. doi:10.1016/j.jorganchem.2004.07.054.
- Dupont, Jairton; Consorti, Crestina S.; Spencer, John (2005-06-01). "The Potential of Palladacycles: More Than Just Precatalysts". Chemical Reviews. 105 (6): 2527–2572. doi:10.1021/cr030681r. ISSN 0009-2665. PMID 15941221.
Bruneau, Alexandre; Roche, Maxime; Alami, Mouad; Messaoudi, Samir (2015-02-06). "2-Aminobiphenyl Palladacycles: The "Most Powerful" Precatalysts in C–C and C–Heteroatom Cross-Couplings". ACS Catalysis. 5 (2): 1386–1396. doi:10.1021/cs502011x. ISSN 2155-5435.
References
- ↑ Cope, Arthur C.; Siekman, Robert W. (July 1965). "Formation of Covalent Bonds from Platinum or Palladium to Carbon by Direct Substitution". Journal of the American Chemical Society. 87 (14): 3272–3273. doi:10.1021/ja01092a063. ISSN 0002-7863.
- ↑ Herrmann, Wolfgang A.; Brossmer, Christoph; Reisinger, Claus-Peter; Riermeier, Thomas H.; Öfele, Karl; Beller, Matthias (August 1997). "Palladacycles: Efficient New Catalysts for the Heck Vinylation of Aryl Halides". Chemistry - A European Journal (in German). 3 (8): 1357–1364. doi:10.1002/chem.19970030823.
- ↑ Trofimenko, S. (1973-06-01). "Cyclopalladation reaction". Inorganic Chemistry. 12 (6): 1215–1221. doi:10.1021/ic50124a001. ISSN 0020-1669.
- ↑ Parshall, George W. (1970-04-01). "Intramolecular aromatic substitution in transition metal complexes". Accounts of Chemical Research. 3 (4): 139–144. doi:10.1021/ar50028a004. ISSN 0001-4842.
- ↑ Rodríguez, Gema; Albrecht, Martin; Schoenmaker, Jeroen; Ford, Alan; Lutz, Martin; Spek, Anthony L.; van Koten, Gerard (May 2002). "Bifunctional Pincer-type Organometallics as Substrates for Organic Transformations and as Novel Building Blocks for Polymetallic Materials". Journal of the American Chemical Society. 124 (18): 5127–5138. doi:10.1021/ja0177657. hdl:1874/14925. ISSN 0002-7863. PMID 11982378. S2CID 31662816.
- ↑ Grove, David M.; Van Koten, Gerard; Louwen, Jaap N.; Noltes, Jan G.; Spek, Anthony L.; Ubbels, Henk J. C. (December 1982). "Trans-2,6-bis[(dimethylamino)methyl]phenyl-N,N',C complexes of palladium(II) and platinum(II). Crystal structure of [PtI[MeC6H3(CH2NMe2)2-o,o']]BF4: a cyclohexadienyl carbonium ion with a .sigma.-bonded metal substituent". Journal of the American Chemical Society. 104 (24): 6609–6616. doi:10.1021/ja00388a022. ISSN 0002-7863.
- ↑ Holton, Robert A.; Kjonaas, Richard A. (June 1977). "Carbopalladation-depalladation of allylic amines and sulfides". Journal of the American Chemical Society. 99 (12): 4177–4179. doi:10.1021/ja00454a057. ISSN 0002-7863.
- ↑ Bruneau, Alexandre; Roche, Maxime; Alami, Mouad; Messaoudi, Samir (2015-02-06). "2-Aminobiphenyl Palladacycles: The "Most Powerful" Precatalysts in C–C and C–Heteroatom Cross-Couplings". ACS Catalysis. 5 (2): 1386–1396. doi:10.1021/cs502011x. ISSN 2155-5435.
- ↑ Lu, Ting-Yi; Xue, Cuihua; Luo, Fen-Tair (February 2003). "Palladium-catalyzed cross-coupling reaction of aryldioxaborolane with 2-bromo-N,N-dimethylacetamide". Tetrahedron Letters. 44 (8): 1587–1590. doi:10.1016/S0040-4039(03)00066-2.
- ↑ Brun, Virginie; Legraverend, Michel; Grierson, David S (September 2002). "Traceless solid-phase synthesis of 2,6,9-trisubstituted purines from resin bound 6-thiopurines". Tetrahedron. 58 (39): 7911–7923. doi:10.1016/S0040-4020(02)00905-5.
- ↑ Prinz, Peter; Lansky, Annegret; Knieriem, Burkhard; de Meijere, Armin; Haumann, Thomas; Boese, Roland; Noltemeyer, Matthias (1997-07-04). "Palladium-Catalyzed Sixfold Alkenylation of Hexabromobenzene: An Interesting Case of Self-Organization". Angewandte Chemie International Edition in English. 36 (12): 1289–1292. doi:10.1002/anie.199712891. ISSN 0570-0833.
- ↑ Zim, Danilo; Buchwald, Stephen L. (July 2003). "An Air and Thermally Stable One- Component Catalyst for the Amination of Aryl Chlorides". Organic Letters. 5 (14): 2413–2415. doi:10.1021/ol034561h. ISSN 1523-7060. PMID 12841743.
- ↑ Holton, Robert A. (November 1977). "Prostaglandin synthesis via carbopalladation". Journal of the American Chemical Society. 99 (24): 8083–8085. doi:10.1021/ja00466a069. ISSN 0002-7863.
- ↑ De Meijere, Armin; Schelper, Michael; Knoke, Mario; Yucel, Baris; Sünnemann, Hans Wolf; Scheurich, René Peter; Arve, Lars (2003-12-07). "Palladium-catalyzed cross-coupling reactions and electrocyclizations—efficient combinations for new cascade reactions". Journal of Organometallic Chemistry. 687 (2): 249–255. doi:10.1016/j.jorganchem.2003.07.007. ISSN 0022-328X.
- ↑ Kapdi, Anant (2019). Palladacycles : catalysis and beyond. Debabrata Maiti (First ed.). Amsterdam. ISBN 978-0-12-816516-4. OCLC 1104998787.
{{cite book}}: CS1 maint: location missing publisher (link)