 | |
| Names | |
|---|---|
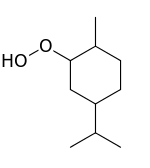
| IUPAC name
2-hydroperoxy-4-methyl-1-propan-2-ylcyclohexane | |
| Other names
Menthyl hydroperoxide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.043.610 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H20O2 | |
| Molar mass | 172.268 g·mol−1 |
| Appearance | Light yellow liquid (50% solution) |
| Odor | Distinct |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Oxidizing, flammable, causes severe burns, explosive decomposition possible above 60°C[1] |
| GHS labelling: | |
   | |
| Danger | |
| H242, H314, H373 | |
| P210, P220, P234, P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P314, P321, P363, P370+P378, P403+P235, P405, P411, P420, P501 | |
| Safety data sheet (SDS) | MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Paramenthane hydroperoxide (PMHP) is an organic peroxide with a distinctive odor. It is used on an industrial scale as a polymerization initiator for emulsion polymerizations. It is usually sold in a light yellow liquid solutions of about 50% strength.[2]
References
- ↑ Lyondellbasell MSDS
- ↑ SASOL (2009). "EC-safety data sheet: paramenthane hydroperoxide (PMHP)," Product MSDS, p. 1.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.