Patient registration is used to correlate the reference position of a virtual 3D dataset gathered by computer medical imaging with the reference position of the patient. This procedure is crucial in computer assisted surgery, in order to insure the reproducitibility of the preoperative registration and the clinical situation during surgery. The use of the term "patient registration" out of this context can lead to a confusion with the procedure of registering a patient into the files of a medical institution.
In computer assisted surgery, the first step is to gather a 3D dataset that reproduces with great accuracy the geometry of the normal and pathological tissues in the region that has to be operated on. This is mainly obtained by using CT or MRI scans of that region. The role of patient registration is to obtain a close-to-ideal reference reproducibility of the dataset – in order to correlate the position (offset) of the gathered dataset with the patient's position during the surgical intervention. Patient registration (1) eliminates the necessity of maintaining the same strict position of the patient during both preoperative scanning and surgery, and (2) provides the surgical robot the necessary reference information to act accurately on the patient, even if he has (been) moved during the intervention.
Application
Patient registration was used mostly in head surgery – oral and maxillofacial surgery, neurosurgery, otolaryngology. With the advent of marker- and markerless-registration, the concept has been extended for abdominal surgery.
Using headframes
The first attempts in 3D mapping of human tissues were made by V. Horsley and R. Clarke in 1906.[1] They have built a rectangular stereotactic headframe that had to be fixed to the head. It was based on cartesian principles and allowed them to accurately and reproductibly guide needle-like electrodes for neurophysiological experiments. They have experimented animals and were able to contribute to the mapping of the cerebellum. Improved versions of the Horsley–Clarke apparatus are still in used today in experimental neurosurgery.
The first stereotactic device for humans was also developed in neurosurgery, by E. Spiegel and H. Wycis in 1947.[2] It was used for surgical treatment of Parkinson's disease and, during time, its applicability was extended for the surgical treatment of tumors, vascular malformations, functional neurosurgery etc. The system was based both on headframes and X-ray images taken for all three planes of space.
Further development of stereotactic surgery was made by Brown, Roberts and Wells in 1980.[3] They have developed a halo ring that was applied on the skull, during a CT scan and neurosurgical interventions. This method provided improved surgical guidance and was in fact the first development of computer guided surgery.
Patient registration for the head area has developed for nearly two decades on the same principle of combining CT scans with mechanical reference devices such as headframes or halo rings. But the clinical experience showed that headgear is very uncomfortable to wear and even impossible to apply on little children, because their lack of cooperation; furthermore, the headframes can create artifacts in preoperative data gathering, or during surgery.
Reference markers
Skin
In 1986, a different approach was developed by Roberts und Strohbehn.[4] They have used as landmarks several markers on the patient's skin both preoperative CT registration, and intraoperatively. This was a new current of the time in patient registration. Still, the method is time-consuming, and the exact reproducitibility of the marker positions is questionable.
Bone
The bony structures can provide a much better stability and reproducibility of the landmarks for patient registration. Based on this concept, a further technique was used: to implant temporary markers into bone structures that are superficial to the skin, under local anestesia.[5] This was also combined with surface markers and CT registration.[6] The technique has the disadvantage of a further minimal surgical procedure of placing the bone implants, with some risk of infection for the patient.

Dental splint markers
Dental splints have been traditionally used for transferring and reproducing 3D reference landmarks for positioning cast models in articulators – in dental prosthetics, orthodontics and orthognathic surgery. By applying several infrared markers on the splints and using an infrared camera, a better registration was obtained.[7]
Markerless patient registration
Anatomical landmarks
The first attempts, based on the identification of anatomical landmarks were made by Caversaccio and Zulliger.[8] The method was based on identifying certain antropometrical points and other anatomical landmarks on the skull, in correlation with the CT registration. But the landmarks cannot be exactly pointed out and reproduced during patient dataset registration and surgery, therefore the method is not precise enough.
Surface registration
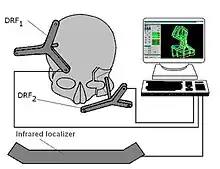
Since 1998, new procedures have been developed by Marmulla and co-workers, using a different approach to the problem.[9][10] Both during CT dataset gathering and surgical intervention, the patient registration was made by registering complete areas and surfaces, instead of distinctive surface markers. This was achieved by using laser scanners and a small guiding transmitter. The precision of the patient registration was significantly improved with this method.
Based on this concept, several registration and navigation systems were built by the same team. The Surgical Segment Navigator (SSN and SSN++) is such a system, developed for the first time for oral and maxillofacial surgery. This system correlates three different coordinate sets: CT data set, surface laser scan data set and the dataset produced by a small guiding transmitter, placed on the patient's head. The Laboratory Unit for Computer-Assisted Surgery (LUCAS) is used for planning surgery in the laboratory. This technological and surgical advance has permitted the elimination of mechanical guidance systems and improved the accuracy of the determinations, and thus the surgical act.
A research group at Ryerson University (now Toronto Metropolitan University) developed a method to use optical topographical imaging (OTI) to create a 3D model of the surface of open surgical sites and perform surface registration to CT and MRI data sets for neurosurgical navigation.[11][12] The OTI technology is being licensed by 7D Surgical for their navigation platform.[12]
References
- ↑ Clarke RH, Horsley V (1906). "On a method of investigating the deep ganglia and tracts of the central nervous system (cerebellum)". British Medical Journal. 2: 1799–1800.
- ↑ Spiegel EA, Wycis HT, Marks M, Lee AJ (October 1947). "Stereotaxic Apparatus for Operations on the Human Brain". Science. 106 (2754): 349–50. Bibcode:1947Sci...106..349S. doi:10.1126/science.106.2754.349. PMID 17777432.
- ↑ Heilbrun MP, Roberts TS, Apuzzo ML, Wells TH, Sabshin JK (August 1983). "Preliminary experience with Brown-Roberts-Wells (BRW) computerized tomography stereotaxic guidance system". Journal of Neurosurgery. 59 (2): 217–22. doi:10.3171/jns.1983.59.2.0217. PMID 6345727.
- ↑ Roberts DW, Strohbehn JW, Hatch JF, Murray W, Kettenberger H (October 1986). "A frameless stereotaxic integration of computerized tomographic imaging and the operating microscope". Journal of Neurosurgery. 65 (4): 545–9. doi:10.3171/jns.1986.65.4.0545. PMID 3531430.
- ↑ Alp MS, Dujovny M, Misra M, Charbel FT, Ausman JI (January 1998). "Head registration techniques for image-guided surgery". Neurological Research. 20 (1): 31–7. doi:10.1080/01616412.1998.11740481. PMID 9471100.
- ↑ Maurer Jr CR, Aboutanos GB, Dawant BM, Margolin RA, Maciunas RJ, Fitzpatrick JM (May 1995). "Registration of CT and MR brain images using a combination of points and surfaces.". Medical Imaging. Vol. 2434. International Society for Optics and Photonics. pp. 109–123.
- ↑ Hassfeld S, Mühling J, Zöller J (February 1995). "Intraoperative navigation in oral and maxillofacial surgery". International Journal of Oral and Maxillofacial Surgery. 24 (1 Pt 2): 111–9. doi:10.1016/s0901-5027(05)80871-9. PMID 7782645.
- ↑ Caversaccio M, Zulliger D, Bächler R, Nolte LP, Häusler R (November 2000). "Practical aspects for optimal registration (matching) on the lateral skull base with an optical frameless computer-aided pointer system". The American Journal of Otology. 21 (6): 863–70. PMID 11078077.
- ↑ Marmulla R, Niederdellmann H (December 1998). "Computer-assisted bone segment navigation". Journal of Cranio-Maxillo-Facial Surgery. 26 (6): 347–59. doi:10.1016/s1010-5182(98)80067-x. PMID 10036650.
- ↑ Marmulla R, Lüth T, Mühling J, Hassfeld S (July 2004). "Markerless laser registration in image-guided oral and maxillofacial surgery". Journal of Oral and Maxillofacial Surgery. 62 (7): 845–51. doi:10.1016/j.joms.2004.01.014. PMID 15218564.
- ↑ Jakubovic R, Guha D, Gupta S, Lu M, Jivraj J, Standish BA, et al. (October 2018). "High Speed, High Density Intraoperative 3D Optical Topographical Imaging with Efficient Registration to MRI and CT for Craniospinal Surgical Navigation". Scientific Reports. 8 (1): 14894. Bibcode:2018NatSR...814894J. doi:10.1038/s41598-018-32424-z. PMC 6173775. PMID 30291261.
- 1 2 Guha D, Jakubovic R, Alotaibi NM, Deorajh R, Gupta S, Fehlings MG, et al. (August 2019). "Optical Topographic Imaging for Spinal Intraoperative 3-Dimensional Navigation in the Cervical Spine: Initial Preclinical and Clinical Feasibility". Clinical Spine Surgery. 32 (7): 303–308. doi:10.1097/BSD.0000000000000795. PMID 30839418.