Pyruvate dehydrogenase lipoamide kinase isozyme 1, mitochondrial is an enzyme that in humans is encoded by the PDK1 gene.[5][6] It codes for an isozyme of pyruvate dehydrogenase kinase (PDK).
Pyruvate dehydrogenase (PDH) is a part of a mitochondrial multienzyme complex that catalyzes the oxidative decarboxylation of pyruvate and is one of the major enzymes responsible for the regulation of homeostasis of carbohydrate fuels in mammals. The enzymatic activity is regulated by a phosphorylation/dephosphorylation cycle. Phosphorylation of PDH by a specific pyruvate dehydrogenase kinase (PDK) results in inactivation.[6]
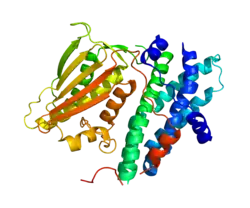
Structure
The mature protein encoded by the PDK4 gene contains 407 amino acids in its sequence. To form the active protein, two of the polypeptide chains come together to form an open conformation.[6] The catalytic domain of PDK1 might exist separately in cells and important for the regulation of the PDK1 substrate. The crystal structural studies suggest that the PIF-pocket is located at the catalytic domain as well.[7]
Function
The Pyruvate Dehydrogenase (PDH) complex must be tightly regulated due to its central role in general metabolism. Within the complex, there are three serine residues on the E1 component that are sites for phosphorylation; this phosphorylation inactivates the complex. In humans, there have been four isozymes of Pyruvate Dehydrogenase Kinase that have been shown to phosphorylate these three sites: PDK1, PDK2, PDK3, and PDK4. PDK1 is the only enzyme capable of phosphorylating the 3rd serine site. When the thiamine pyrophosphatase (TPP) coenzyme is bound, the rates of phosphorylation by all four isozymes are drastically affected; specifically, the incorporation of phosphate groups by PDK1 into sites 2 and 3 is significantly reduced.[8]
Regulation
As the primary regulators of a crucial step in the central metabolic pathway, the pyruvate dehydrogenase family is tightly regulated itself by a myriad of factors. PDK activity has been shown to decrease in individuals consuming a diet that is high in n-3 fatty acids; however, PDH activity remained unaffected.[9] Additionally, PDK1 is inhibited by AZD7545 and dichloroacetic acid (DCA); the mechanism was discovered to be the trifluoromethylpropanamide end of AZD7545 projecting into the lipoyl-binding pocket of PDK1. Dichloroacetic acid was found near the helix bundle in the N-terminal domain of PDK1. Bound DCA promotes local conformational changes that are communicated to both nucleotide-binding and lipoyl-binding pockets of PDK1, leading to the inactivation of kinase activity.[10]
Clinical Significance
PDK1 is relevant in a variety of clinical conditions throughout the body. As PDK1 regulates the PDH complex, it has been proven to be an important regulator in certain cells, including the beta cells within the islets of the pancreas. In order to optimize glucose-stimulated insulin secretion (GSIS), a primary function of the pancreas, a low PDK1 activity must be maintained to keep PDH in a dephosphorylated and active state.[11] Maintaining low PDK1 levels has also proven to be beneficial in certain regions of the brain, as it confers a high tolerance to amyloid beta, a metabolite that is directly correlated with the development of Alzheimer’s disease.[12]
Cancer
The ubiquitous role of this gene lends itself to being involved in a variety of disease pathologies, including cancer. PDK1 mRNA expression is significantly associated with tumor progression; in fact, the presence of PDK1 can serve as a prognostic marker, indicating the level of success a patient can achieve. Specifically, this may serve as a biomarker in patients with gastric cancer. In coordination, the inhibitor dichloroacetic acid may be used in the future as a treatment option for patients with this type of cancer.[13] PDK1, as it regulates hypoxia and lactate production, is associated with a poor outcome in patients with head and neck squamous cancer.[14][15] The buildup of glycolytic metabolites may promote Hypoxia-Inducing Factor (HIF) activation, which creates a feed-forward loop for malignancy progression. As such, using HIF-1 as a metabolite to regulate PDK1 is seen as another potential therapy, either on its own or in tandem with other therapies, for this type of cancer.[16][17] In a further developed study, combined PDK1 and CHK1 inhibition was shown to be required to kill glioblastoma stem-like cells in vitro and in vivo.[18]
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000152256 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000006494 - Ensembl, May 2017
- ↑ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ Gudi R, Bowker-Kinley MM, Kedishvili NY, Zhao Y, Popov KM (Jan 1996). "Diversity of the pyruvate dehydrogenase kinase gene family in humans". J Biol Chem. 270 (48): 28989–94. doi:10.1074/jbc.270.48.28989. PMID 7499431.
- 1 2 3 "Entrez Gene: PDK1 pyruvate dehydrogenase kinase, isozyme 1".
- ↑ Park J, Li Y, Kim SH, Kong G, Shrestha R, Tran Q, Hong J, Hur GM, Hemmings BA, Koo BS, Park J (Nov 2013). "Characterization of fragmented 3-phosphoinsitide-dependent protein kinase-1 (PDK1) by phosphosite-specific antibodies". Life Sciences. 93 (18–19): 700–6. doi:10.1016/j.lfs.2013.09.007. PMID 24044887.
- ↑ Kolobova E, Tuganova A, Boulatnikov I, Popov KM (Aug 2001). "Regulation of pyruvate dehydrogenase activity through phosphorylation at multiple sites". The Biochemical Journal. 358 (Pt 1): 69–77. doi:10.1042/0264-6021:3580069. PMC 1222033. PMID 11485553.
- ↑ Turvey EA, Heigenhauser GJ, Parolin M, Peters SJ (Jan 2005). "Elevated n-3 fatty acids in a high-fat diet attenuate the increase in PDH kinase activity but not PDH activity in human skeletal muscle". Journal of Applied Physiology. 98 (1): 350–5. doi:10.1152/japplphysiol.00604.2005. PMID 15591305.
- ↑ Kato M, Li J, Chuang JL, Chuang DT (Aug 2007). "Distinct structural mechanisms for inhibition of pyruvate dehydrogenase kinase isoforms by AZD7545, dichloroacetate, and radicicol". Structure. 15 (8): 992–1004. doi:10.1016/j.str.2007.07.001. PMC 2871385. PMID 17683942.
- ↑ Krus U, Kotova O, Spégel P, Hallgard E, Sharoyko VV, Vedin A, Moritz T, Sugden MC, Koeck T, Mulder H (Jul 2010). "Pyruvate dehydrogenase kinase 1 controls mitochondrial metabolism and insulin secretion in INS-1 832/13 clonal beta-cells" (PDF). The Biochemical Journal. 429 (1): 205–13. doi:10.1042/BJ20100142. PMID 20415663.
- ↑ Newington JT, Rappon T, Albers S, Wong DY, Rylett RJ, Cumming RC (Oct 2012). "Overexpression of pyruvate dehydrogenase kinase 1 and lactate dehydrogenase A in nerve cells confers resistance to amyloid β and other toxins by decreasing mitochondrial respiration and reactive oxygen species production". The Journal of Biological Chemistry. 287 (44): 37245–58. doi:10.1074/jbc.M112.366195. PMC 3481323. PMID 22948140.
- ↑ Hur H, Xuan Y, Kim YB, Lee G, Shim W, Yun J, Ham IH, Han SU (Jan 2013). "Expression of pyruvate dehydrogenase kinase-1 in gastric cancer as a potential therapeutic target". International Journal of Oncology. 42 (1): 44–54. doi:10.3892/ijo.2012.1687. PMC 3583751. PMID 23135628.
- ↑ Wigfield SM, Winter SC, Giatromanolaki A, Taylor J, Koukourakis ML, Harris AL (Jun 2008). "PDK-1 regulates lactate production in hypoxia and is associated with poor prognosis in head and neck squamous cancer". British Journal of Cancer. 98 (12): 1975–84. doi:10.1038/sj.bjc.6604356. PMC 2441961. PMID 18542064.
- ↑ Hitosugi T, Fan J, Chung TW, Lythgoe K, Wang X, Xie J, Ge Q, Gu TL, Polakiewicz RD, Roesel JL, Chen GZ, Boggon TJ, Lonial S, Fu H, Khuri FR, Kang S, Chen J (Dec 2011). "Tyrosine phosphorylation of mitochondrial pyruvate dehydrogenase kinase 1 is important for cancer metabolism". Molecular Cell. 44 (6): 864–77. doi:10.1016/j.molcel.2011.10.015. PMC 3246218. PMID 22195962.
- ↑ Kim JW, Tchernyshyov I, Semenza GL, Dang CV (Mar 2006). "HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia". Cell Metabolism. 3 (3): 177–85. doi:10.1016/j.cmet.2006.02.002. PMID 16517405.
- ↑ McFate T, Mohyeldin A, Lu H, Thakar J, Henriques J, Halim ND, Wu H, Schell MJ, Tsang TM, Teahan O, Zhou S, Califano JA, Jeoung NH, Harris RA, Verma A (Aug 2008). "Pyruvate dehydrogenase complex activity controls metabolic and malignant phenotype in cancer cells". The Journal of Biological Chemistry. 283 (33): 22700–8. doi:10.1074/jbc.M801765200. PMC 2504897. PMID 18541534.
- ↑ Signore M, Pelacchi F, di Martino S, Runci D, Biffoni M, Giannetti S, Morgante L, De Majo M, Petricoin EF, Stancato L, Larocca LM, De Maria R, Pallini R, Ricci-Vitiani L (8 May 2014). "Combined PDK1 and CHK1 inhibition is required to kill glioblastoma stem-like cells in vitro and in vivo". Cell Death & Disease. 5 (5): e1223. doi:10.1038/cddis.2014.188. PMC 4047898. PMID 24810059.
Further reading
- Sugden MC, Holness MJ (2003). "Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs". Am. J. Physiol. Endocrinol. Metab. 284 (5): E855–62. doi:10.1152/ajpendo.00526.2002. PMID 12676647.
- Liu S, Baker JC, Andrews PC, Roche TE (1995). "Recombinant expression and evaluation of the lipoyl domains of the dihydrolipoyl acetyltransferase component of the human pyruvate dehydrogenase complex". Arch. Biochem. Biophys. 316 (2): 926–40. doi:10.1006/abbi.1995.1124. PMID 7864652.
- Kolobova E, Tuganova A, Boulatnikov I, Popov KM (2001). "Regulation of pyruvate dehydrogenase activity through phosphorylation at multiple sites". Biochem. J. 358 (Pt 1): 69–77. doi:10.1042/0264-6021:3580069. PMC 1222033. PMID 11485553.
- Korotchkina LG, Patel MS (2001). "Site specificity of four pyruvate dehydrogenase kinase isoenzymes toward the three phosphorylation sites of human pyruvate dehydrogenase". J. Biol. Chem. 276 (40): 37223–9. doi:10.1074/jbc.M103069200. PMID 11486000.
- Tuganova A, Boulatnikov I, Popov KM (2002). "Interaction between the individual isoenzymes of pyruvate dehydrogenase kinase and the inner lipoyl-bearing domain of transacetylase component of pyruvate dehydrogenase complex". Biochem. J. 366 (Pt 1): 129–36. doi:10.1042/BJ20020301. PMC 1222743. PMID 11978179.