 | |
| Names | |
|---|---|
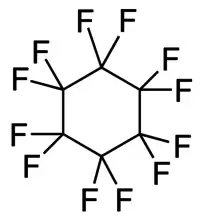
| Preferred IUPAC name
Dodecafluorocyclohexane | |
| Other names
1,1,2,2,3,3,4,4,5,5,6,6-Dodecafluorocyclohexane, Cyclohexane, dodecafluoro- | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.005.993 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6F12 | |
| Molar mass | 300.047 g·mol−1 |
| Appearance | clear, waxy solid |
| Density | 1.684 g/cm3 |
| Melting point | 52 °C (126 °F; 325 K)[1] |
| Boiling point | 59–60 °C (138–140 °F; 332–333 K) |
| Solubility | Miscible with organic compounds |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Irritant |
| GHS labelling: | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| Safety data sheet (SDS) | MSDS |
| Related compounds | |
Related compounds |
Fluorocarbon |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Perfluorocyclohexane or dodecafluorocyclohexane is a chemical which belongs to the class of fluorocarbons, sometimes referred to as perfluorocarbons or PFCs. Fluorocarbons and their derivatives are useful fluoropolymers, refrigerants, solvents, and anesthetics.
Synthesis
Perfluorocyclohexane can be synthesized by fluorination of cyclohexane.[2]
Properties
Perfluorocyclohexane is chemically inert and thermally stable. It is a relatively non-toxic, clear, waxy solid, which has a high vapor pressure and therefore sublimes readily at room temperature.
The molecule predominantly exists in its chair conformation, in which it possesses D3d molecular symmetry.
References
- ↑ Sander, M.; Blöchl, W. (January 1965). "Herstellung von Perfluoralkanen und Perfluorcycloalkanen". Chemie Ingenieur Technik (in German). 37 (1): 7–13. doi:10.1002/cite.330370103.
- ↑ Sandford G. (2003). "Perfluoroalkanes". Tetrahedron. 59 (4): 437–454. doi:10.1016/s0040-4020(02)01568-5.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.