 | |
| Names | |
|---|---|
| Other names
pentafluoropropionic acid perfluoropropanoic acid PFPrA C3 PFCA | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.006.384 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3HF5O2 | |
| Molar mass | 164.031 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.561 g/mL |
| Boiling point | 96–97 °C (205–207 °F; 369–370 K) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
corrosive |
| GHS labelling:[1] | |
  | |
| Danger | |
| H314, H332 | |
| P280, P301+P330+P331, P305+P351+P338, P310 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
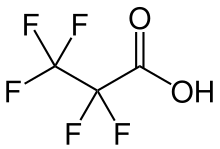
Perfluoropropionic acid (PFPrA[2]) or pentafluoropropionic acid is the perfluoroalkyl carboxylic acid with the formula CF3CF2CO2H. It is a colorless liquid that is strongly acidic and soluble in water and polar organic solvents.
A convenient, safe method for generating tetrafluoroethylene is the pyrolysis of the sodium salt of pentafluoropropionic acid:[3]
- C2F5CO2Na → C2F4 + CO2 + NaF
References
- ↑ "Pentafluoropropionic acid, 97% – Perfluoropropionic acid – A12791". Alfa Aesar. Retrieved 2022-03-23.
- ↑ Buck, Robert C; Franklin, James; Berger, Urs; Conder, Jason M; Cousins, Ian T; de Voogt, Pim; Jensen, Allan Astrup; Kannan, Kurunthachalam; Mabury, Scott A; van Leeuwen, Stefan PJ (2011). "Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins". Integrated Environmental Assessment and Management. 7 (4): 513–541. doi:10.1002/ieam.258. PMC 3214619. PMID 21793199.
- ↑ Hercules, Daniel A.; Parrish, Cameron A.; Sayler, Todd S.; Tice, Kevin T.; Williams, Shane M.; Lowery, Lauren E.; Brady, Michael E.; Coward, Robert B.; Murphy, Justin A.; Hey, Trevyn A.; Scavuzzo, Anthony R.; Rummler, Lucy M.; Burns, Emory G.; Matsnev, Andrej V.; Fernandez, Richard E.; McMillen, Colin D.; Thrasher, Joseph S. (2017). "Preparation of tetrafluoroethylene from the pyrolysis of pentafluoropropionate salts". Journal of Fluorine Chemistry. 196: 107–116. doi:10.1016/j.jfluchem.2016.10.004.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.