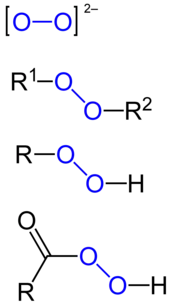
Types of peroxides, from top to bottom: peroxide ion, organic peroxide, organic hydroperoxide, peracid. The peroxide group is marked in
blue. R, R1 and R2 mark hydrocarbon moieties.
In chemistry, peroxides are a group of compounds with the structure R−O−O−R, where R is any element.[1][2] The O−O group in a peroxide is called the peroxide group or peroxy group (sometimes called peroxo group or peroxyl group). The nomenclature is somewhat variable,[3] and the term was introduced by Thomas Thomson in 1804 for an oxide with the greatest quantity of oxygen.[4]
The most common peroxide is hydrogen peroxide (H2O2), colloquially known simply as "peroxide". It is marketed as solutions in water at various concentrations. Many organic peroxides are known as well.
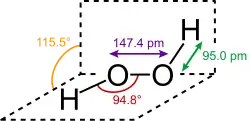
Structure and dimensions of H2O2 in gas phase.
In addition to hydrogen peroxide, some other major classes of peroxides are:
- Peroxy acids, the peroxy derivatives of many familiar acids, examples being peroxymonosulfuric acid and peracetic acid, and their salts, one example of which is potassium peroxydisulfate.
- Main group peroxides, compounds with the linkage E−O−O−E (E = main group element).
- Metal peroxides, examples being barium peroxide (BaO2), sodium peroxide (Na2O2) and zinc peroxide (ZnO2).
- Organic peroxides, compounds with the linkage C−O−O−C or C−O−O−H. One example is tert-butylhydroperoxide.
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 978-0-471-72091-1
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "peroxides". doi:10.1351/goldbook.P04510
- ↑ Harper, Douglas. "peroxide". Online Etymology Dictionary.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.