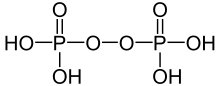
 | |
| Names | |
|---|---|
| Other names
peroxodiphosphoric acid | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| H4P2O8 | |
| Molar mass | 193.97 g/mol |
| Related compounds | |
Related compounds |
peroxymonophosphoric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Peroxydiphosphoric acid (H4P2O8) is an oxyacid of phosphorus. Its salts are known as peroxydiphosphates. It is one of two peroxyphosphoric acids, along with peroxymonophosphoric acid.
History
Both peroxyphosphoric acids were first synthesized and characterized in 1910 by Julius Schmidlin and Paul Massini,[1] where peroxydiphosphoric acid was obtained in poor yields from the reaction between diphosphoric acid and highly-concentrated hydrogen peroxide.
Preparation
Peroxydiphosphoric acid can be prepared by the reaction between phosphoric acid and fluorine, with peroxymonophosphoric acid being a by-product.[2]
The compound is not commercially available and must be prepared as needed.[2] Peroxodiphosphates can be obtained by electrolysis of phosphate solutions.[3]
Properties
Peroxydiphosphoric acid is a tetraprotic acid, with acid dissociation constants given by pKa1 ≈ −0.3, pKa2 ≈ 0.5, pKa3 = 5.2 and pKa4 = 7.6.[4] In aqueous solution, it disproportionates upon heating to peroxymonophosphoric acid and phosphoric acid.[5]
References
- ↑ Schmidlin, Julius; Massini, Paul (1910). "Phosphormonopersäure und Überphosphorsäure". Ber. Dtsch. Chem. Ges. 43 (1): 1162–1171. doi:10.1002/cber.191004301195.
- 1 2 Harald, Jakob; Leininger, Stefan; Lehmann, Thomas; Jacobi, Sylvia; Gutewort, Sven (2007). "Peroxo Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Wiley‐VCH Verlag. pp. 310–311. doi:10.1002/14356007.a19_177.pub2. ISBN 9783527306732.
- ↑ Riedel, Erwin (2004). Anorganische Chemie (6 ed.). Berlin/New York: de Gruyter. p. 493.
- ↑ Crutchfield, Marvin M.; Edwards, John O. (1960). "The Acidity and Complexes of Peroxydiphosphoric Acid". J. Am. Chem. Soc. 82 (14): 3533–3537. doi:10.1021/ja01499a015.
- ↑ Kolditz, Lothar (1983). Anorganische Chemie. Vol. 1. Berlin: Deutscher Verlag der Wissenschaften. p. 437.