In chemistry, phosphorochloridites are a class of organophosphorus compound with the formula (RO)2PCl (R = organic substituent). They are pyramidal in shape, akin to regular phosphites (P(OR)3). They are usually colorless and sensitive toward hydrolysis and, to some extent, oxidation to the corresponding phosphorochloridates ((RO)2P(O)Cl).
Synthesis and reactions
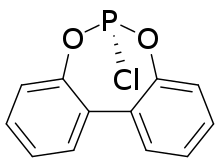
Phosphorochloridites are produced by partial alcoholysis of phosphorus trichloride, which proceeds stepwise:[1]
- PCl3 + ROH → HCl + (RO)PCl2 (phosphochloridite)
- (RO)PCl2 + ROH → HCl + (RO)2PCl (phosphodichloridite)
- (RO)2PCl + ROH → HCl + (RO)3P (phosphite)
These reactions are readily controlled with aromatic diols, such as binaphthol and 2,2'-biphenol.
Phosphorochloridites are precursors to diphosphite ligands. When combined with rhodium precursors such as Rh(acac)(CO)2, these diphosphite ligands afford catalysts that are used industrially for the hydroformylation of alkenes.[2] it and related ligands have become popular in hydroformylation catalysis.[3][4]
References
- ↑ Gual, Aitor; Godard, Cyril; de la Fuente, Verónica; Castillón, Sergio (2012). "Design and Synthesis of Phosphite Ligands for Homogeneous Catalysis". Phosphorus(III) Ligands in Homogeneous Catalysis: Design and Synthesis. pp. 81–131. doi:10.1002/9781118299715.ch3. ISBN 9781118299715.
- ↑ Billig, Ernst; Abatjoglou, Anthony G.; Bryant, David R. (1988). "Homogeneous Rhodium Carbonyl Compound-Phosphite Ligand Catalysts and Process for Olefin Hydroformylation". US 4769498 a 19880906 to Union Carbide.
- ↑ Cuny, Gregory D.; Buchwald, Stephen L. (1993). "Practical, High-Yield, Regioselective, Rhodium-Catalyzed Hydroformylation of Functionalized α-olefins". Journal of the American Chemical Society. 115 (5): 2066–2068. doi:10.1021/ja00058a079.
- ↑ Van Rooy, Annemiek; Kamer, Paul C. J.; Van Leeuwen, Piet W. N. M.; Goubitz, Kees; Fraanje, Jan; Veldman, Nora; Spek, Anthony L. (1996). "Bulky Diphosphite-Modified Rhodium Catalysts: Hydroformylation and Characterization". Organometallics. 15 (2): 835–847. doi:10.1021/OM950549K.