 | |
| Names | |
|---|---|
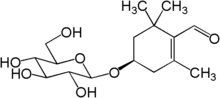
| IUPAC name
(4R)-4-(β-D-Glucopyranosyloxy)-2,6,6-trimethylcyclohex-1-ene-1-carbaldehyde | |
| Systematic IUPAC name
(4R)-2,6,6-Trimethyl-4-{[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}cyclohex-1-ene-1-carbaldehyde | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H26O7 | |
| Molar mass | 330.37 g/mol |
| Density | 1.31 g/mL |
| Melting point | 154 to 156 °C (309 to 313 °F; 427 to 429 K) |
| Boiling point | 520.4 °C (968.7 °F; 793.5 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Picrocrocin is a monoterpene glycoside precursor of safranal. It is found in the spice saffron, which comes from the crocus flower.[1] Picrocrocin has a bitter taste, and is the chemical most responsible for the taste of saffron.
During the drying process, picrocrocin liberates the aglycone (HTCC, C10H16O2) due to the action of the enzyme glucosidase. The aglycone is then transformed to safranal by dehydration. Picrocrocin is a degradation product of the carotenoid zeaxanthin.
References
- ↑ Caballero-Ortega H, Pereda-Miranda R, Abdullaev FI (2007). "HPLC quantification of major active components from 11 different saffron (Crocus sativus L.) sources". Food Chemistry. 100 (3): 1126–1131. doi:10.1016/j.foodchem.2005.11.020.
- Pfander, H.; Schurtenberger, H. (1982). "Biosynthesis of C20-carotenoids in Crocus sativus". Phytochemistry. 21 (5): 1039–1042. Bibcode:1982PChem..21.1039P. doi:10.1016/S0031-9422(00)82412-7.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.