 | |
| Names | |
|---|---|
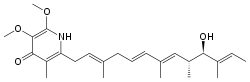
| Preferred IUPAC name
2-[(2E,5E,7E,9R,10R,11E)-10-Hydroxy-3,7,9,11-tetramethyltrideca-2,5,7,11-tetraen-1-yl]-5,6-dimethoxy-3-methylpyridin-4(1H)-one | |
| Other names
Piericidin A1, AR-054 | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.162.726 |
| MeSH | Piericidin+A |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C25H37NO4 | |
| Molar mass | 415.566 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Piericidin A is an antibiotic agent.[1] It was discovered from Streptomyces mobaraensis. Being an inhibitor of NADH dehydrogenase, it inhibits electron transfer; its structure resembles that of the ubiquinone, therefore it competes with QB for binding sites in NADH dehydrogenase as well as Photosystem II.
References
- ↑ "MeSH Record of Piericidin A". U.S. National Library of Medicine, NIH. Retrieved 2018-05-22.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.