 | |
| Clinical data | |
|---|---|
| Trade names | Jeselhy |
| Other names | TAS 116 |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
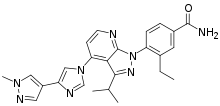
| Formula | C25H26N8O |
| Molar mass | 454.538 g·mol−1 |
| 3D model (JSmol) | |
| |
Pimitespib (trade name Jeselhy) is a drug for the treatment of gastrointestinal stromal tumors.[1] It was discovered and developed by Taiho Pharmaceutical Co.[2] The mechanism of action of pimitespib involves inhibition of heat shock protein 90 (Hsp90).[3]
In June 2022, it received approval in Japan for gastrointestinal stromal tumors that have progressed after chemotherapy.[4]
References
- ↑ "Pimitespib - Taiho Pharmaceutical". Adis Insight.
- ↑ "Taiho Pharmaceutical Obtains Approval to Manufacture and Market HSP90 inhibitor Jeselhy® Tablets 40 mg (Pimitespib) for Gastrointestinal Stromal Tumor (GIST)" (Press release). Taiho Pharmaceutical. June 20, 2022.
- ↑ Kurokawa Y, Honma Y, Sawaki A, Naito Y, Iwagami S, Komatsu Y, et al. (June 2022). "Pimitespib in patients with advanced gastrointestinal stromal tumor (CHAPTER-GIST-301): a randomized, double-blind, placebo-controlled phase III trial". Annals of Oncology. 33 (9): 959–967. doi:10.1016/j.annonc.2022.05.518. PMID 35688358. S2CID 249532381.
- ↑ Hoy SM (August 2022). "Pimitespib: First Approval". Drugs. 82 (13): 1413–1418. doi:10.1007/s40265-022-01764-6. PMID 35986838. S2CID 251672805.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.