 | |
| Names | |
|---|---|
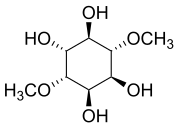
| IUPAC name
1D-1,4-Di-O-methyl-chiro-inositol | |
| Systematic IUPAC name
(1R,2R,3R,4S,5R,6S)-3,6-Dimethoxycyclohexane-1,2,4,5-tetrol | |
| Other names
1,4-Di-O-Methyl-D-chiro-inositol | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H16O6 | |
| Molar mass | 208.210 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Pinpollitol is a cyclitol. It is a di-O-methyl-(+)-chiro-inositol that can be isolated from Pinus radiata.[1]
References
- ↑ Gallagher, R (1975). "(+)-Pinpollitol: A di-O-methyl D-(+)-chiro-inositol from Pinus radiata". Phytochemistry. 14 (3): 755–757. doi:10.1016/0031-9422(75)83029-9.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.