| Cytochrome b6f complex | |
|---|---|
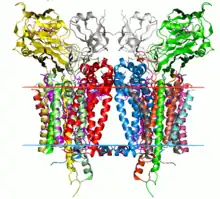 Crystal structure of the cytochrome b6f complex from C. reinhardtii (1q90). Hydrocarbon boundaries of the lipid bilayer are shown by red and blue lines (thylakoid space side and stroma side, respectively). | |
| Identifiers | |
| Symbol | B6F |
| TCDB | 3.D.3 |
| OPM superfamily | 92 |
| OPM protein | 4pv1 |
| Membranome | 258 |
| Cytochrome b6f complex | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 7.1.1.6 | ||||||||
| CAS no. | 79079-13-3 | ||||||||
| Alt. names | Plastoquinol/plastocyanin reductase | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
The cytochrome b6f complex (plastoquinol/plastocyanin reductase or plastoquinol/plastocyanin oxidoreductase; EC 7.1.1.6) is an enzyme found in the thylakoid membrane in chloroplasts of plants, cyanobacteria, and green algae, that catalyzes the transfer of electrons from plastoquinol to plastocyanin:
- plastoquinol + 2 oxidized plastocyanin + 2 H+ [side 1] plastoquinone + 2 reduced plastocyanin + 4 H+ [side 2].[1]
The reaction is analogous to the reaction catalyzed by cytochrome bc1 (Complex III) of the mitochondrial electron transport chain. During photosynthesis, the cytochrome b6f complex is one step along the chain that transfers electrons from Photosystem II to Photosystem I, and at the same time pumps protons into the thylakoid space, contributing to the generation of an electrochemical (energy) gradient[2] that is later used to synthesize ATP from ADP.
Enzyme structure
The cytochrome b6f complex is a dimer, with each monomer composed of eight subunits.[3] These consist of four large subunits: a 32 kDa cytochrome f with a c-type cytochrome, a 25 kDa cytochrome b6 with a low- and high-potential heme group, a 19 kDa Rieske iron-sulfur protein containing a [2Fe-2S] cluster, and a 17 kDa subunit IV; along with four small subunits (3-4 kDa): PetG, PetL, PetM, and PetN.[3][4] The total molecular weight is 217 kDa.
The crystal structures of cytochrome b6f complexes from Chlamydomonas reinhardtii, Mastigocladus laminosus, and Nostoc sp. PCC 7120 have been determined.[2][5][6][7][8][9]
The core of the complex is structurally similar to the cytochrome bc1 core. Cytochrome b6 and subunit IV are homologous to cytochrome b,[10] and the Rieske iron-sulfur proteins of the two complexes are homologous.[11] However, cytochrome f and cytochrome c1 are not homologous.[12]
Cytochrome b6f contains seven prosthetic groups.[13][14] Four are found in both cytochrome b6f and bc1: the c-type heme of cytochrome c1 and f, the two b-type hemes (bp and bn) in bc1 and b6f, and the [2Fe-2S] cluster of the Rieske protein. Three unique prosthetic groups are found in cytochrome b6f: chlorophyll a, β-carotene, and heme cn (also known as heme x).[5]
The inter-monomer space within the core of the cytochrome b6f complex dimer is occupied by lipids,[9] which provides directionality to heme-heme electron transfer through modulation of the intra-protein dielectric environment.[15]
| ||||||||||||||||||||
Biological function
_cyt6bf_mutant.jpg.webp)
In photosynthesis, the cytochrome b6f complex functions to mediate the transfer of electrons and of energy between the two photosynthetic reaction center complexes, Photosystem II and Photosystem I, while transferring protons from the chloroplast stroma across the thylakoid membrane into the lumen.[2] Electron transport via cytochrome b6f is responsible for creating the proton gradient that drives the synthesis of ATP in chloroplasts.[4]
In a separate reaction, the cytochrome b6f complex plays a central role in cyclic photophosphorylation, when NADP+ is not available to accept electrons from reduced ferredoxin.[16] This cycle, driven by the energy of P700+, contributes to the creation of a proton gradient that can be used to drive ATP synthesis. It has been shown that this cycle is essential for photosynthesis,[17] helping to maintain the proper ratio of ATP/NADPH production for carbon fixation.[18][19]
The p-side quinol deprotonation-oxidation reactions within the cytochrome b6f complex have been implicated in the generation of reactive oxygen species.[20] An integral chlorophyll molecule located within the quinol oxidation site has been suggested to perform a structural, non-photochemical function in enhancing the rate of formation of the reactive oxygen species, possibly to provide a redox-pathway for intra-cellular communication.[21]
Reaction mechanism
The cytochrome b6f complex is responsible for "non-cyclic" (1) and "cyclic" (2) electron transfer between two mobile redox carriers, plastoquinol (QH2) and plastocyanin (Pc):
| H2O | → | photosystem II | → | QH2 | → | Cyt b6f | → | Pc | → | photosystem I | → | NADPH | (1) |
| QH2 | → | Cyt b6f | → | Pc | → | photosystem I | → | Q | (2) | ||||
Cytochrome b6f catalyzes the transfer of electrons from plastoquinol to plastocyanin, while pumping two protons from the stroma into the thylakoid lumen:
- QH2 + 2Pc(Cu2+) + 2H+ (stroma) → Q + 2Pc(Cu+) + 4H+ (lumen)[16]
This reaction occurs through the Q cycle as in Complex III.[22] Plastoquinol acts as the electron carrier, transferring its two electrons to high- and low-potential electron transport chains (ETC) via a mechanism called electron bifurcation.[23] The complex contains up to three plastoquinone molecules that form an electron transfer network that are responsible for the operation of the Q cycle and its redox-sensing and catalytic functions in photosynthesis.[24]
Q cycle
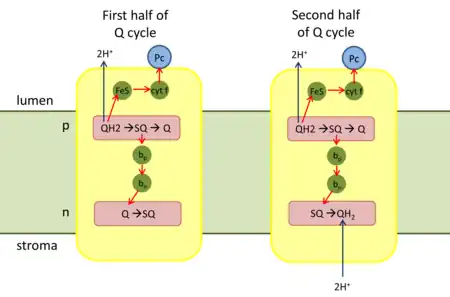
First half of Q cycle
- QH2 binds to the positive 'p' side (lumen side) of the complex. It is oxidized to a semiquinone (SQ) by the iron-sulfur center (high-potential ETC) and releases two protons to the thylakoid lumen.
- The reduced iron-sulfur center transfers its electron through cytochrome f to Pc.
- In the low-potential ETC, SQ transfers its electron to heme bp of cytochrome b6.
- Heme bp then transfers the electron to heme bn.
- Heme bn reduces Q with one electron to form SQ.
Second half of Q cycle
- A second QH2 binds to the complex.
- In the high-potential ETC, one electron reduces another oxidized Pc.
- In the low-potential ETC, the electron from heme bn is transferred to SQ, and the completely reduced Q2− takes up two protons from the stroma to form QH2.
- The oxidized Q and the reduced QH2 that has been regenerated diffuse into the membrane.
Cyclic electron transfer
Unlike Complex III, cytochrome b6f catalyzes another electron transfer reaction that is central to cyclic photophosphorylation. The electron from ferredoxin (Fd) is transferred to plastoquinone and then the cytochrome b6f complex to reduce plastocyanin, which is reoxidized by P700 in Photosystem I.[25] The exact mechanism of the reduction of plastoquinone by ferredoxin is still under investigation. One proposal is that there exists a ferredoxin:plastoquinone-reductase or an NADP dehydrogenase.[25] Since heme x does not appear to be required for the Q cycle and is not found in Complex III, it has been proposed that it is used for cyclic photophosphorylation by the following mechanism:[23][26]
- Fd (red) + heme x (ox) → Fd (ox) + heme x (red)
- heme x (red) + Fd (red) + Q + 2H+ → heme x (ox) + Fd (ox) + QH2
References
- ↑ ExplorEnz: EC 7.1.1.6
- 1 2 3 Hasan SS, Yamashita E, Baniulis D, Cramer WA (Mar 2013). "Quinone-dependent proton transfer pathways in the photosynthetic cytochrome b6f complex". Proceedings of the National Academy of Sciences of the United States of America. 110 (11): 4297–302. doi:10.1073/pnas.1222248110. PMC 3600468. PMID 23440205.
- 1 2 Whitelegge JP, Zhang H, Aguilera R, Taylor RM, Cramer WA (Oct 2002). "Full subunit coverage liquid chromatography electrospray ionization mass spectrometry (LCMS+) of an oligomeric membrane protein: cytochrome b(6)f complex from spinach and the cyanobacterium Mastigocladus laminosus". Molecular & Cellular Proteomics. 1 (10): 816–27. doi:10.1074/mcp.m200045-mcp200. PMID 12438564.
- 1 2 Voet DJ, Voet JG (2011). Biochemistry. New York, NY: Wiley, J. ISBN 978-0-470-57095-1.
- 1 2 Stroebel D, Choquet Y, Popot JL, Picot D (Nov 2003). "An atypical haem in the cytochrome b(6)f complex". Nature. 426 (6965): 413–8. doi:10.1038/nature02155. PMID 14647374. S2CID 130033.
- ↑ Yamashita E, Zhang H, Cramer WA (Jun 2007). "Structure of the cytochrome b6f complex: quinone analogue inhibitors as ligands of heme cn". Journal of Molecular Biology. 370 (1): 39–52. doi:10.1016/j.jmb.2007.04.011. PMC 1993820. PMID 17498743.
- ↑ Baniulis D, Yamashita E, Whitelegge JP, Zatsman AI, Hendrich MP, Hasan SS, Ryan CM, Cramer WA (Apr 2009). "Structure-Function, Stability, and Chemical Modification of the Cyanobacterial Cytochrome b6f Complex from Nostoc sp. PCC 7120". The Journal of Biological Chemistry. 284 (15): 9861–9. doi:10.1074/jbc.M809196200. PMC 2665108. PMID 19189962.
- ↑ Hasan SS, Stofleth JT, Yamashita E, Cramer WA (Apr 2013). "Lipid-induced conformational changes within the cytochrome b6f complex of oxygenic photosynthesis". Biochemistry. 52 (15): 2649–54. doi:10.1021/bi301638h. PMC 4034689. PMID 23514009.
- 1 2 Hasan SS, Cramer WA (Jul 2014). "Internal lipid architecture of the hetero-oligomeric cytochrome b6f complex". Structure. 22 (7): 1008–15. doi:10.1016/j.str.2014.05.004. PMC 4105968. PMID 24931468.
- ↑ Widger WR, Cramer WA, Herrmann RG, Trebst A (Feb 1984). "Sequence homology and structural similarity between cytochrome b of mitochondrial complex III and the chloroplast b6-f complex: position of the cytochrome b hemes in the membrane". Proceedings of the National Academy of Sciences of the United States of America. 81 (3): 674–8. doi:10.1073/pnas.81.3.674. PMC 344897. PMID 6322162.
- ↑ Carrell CJ, Zhang H, Cramer WA, Smith JL (Dec 1997). "Biological identity and diversity in photosynthesis and respiration: structure of the lumen-side domain of the chloroplast Rieske protein". Structure. 5 (12): 1613–25. doi:10.1016/s0969-2126(97)00309-2. PMID 9438861.
- ↑ Martinez SE, Huang D, Szczepaniak A, Cramer WA, Smith JL (Feb 1994). "Crystal structure of chloroplast cytochrome f reveals a novel cytochrome fold and unexpected heme ligation". Structure. 2 (2): 95–105. doi:10.1016/s0969-2126(00)00012-5. PMID 8081747.
- ↑ Baniulis D, Yamashita E, Zhang H, Hasan SS, Cramer WA (2008). "Structure-function of the cytochrome b6f complex". Photochemistry and Photobiology. 84 (6): 1349–58. doi:10.1111/j.1751-1097.2008.00444.x. PMID 19067956. S2CID 44992397.
- ↑ Cramer WA, Zhang H, Yan J, Kurisu G, Smith JL (May 2004). "Evolution of photosynthesis: time-independent structure of the cytochrome b6f complex". Biochemistry. 43 (20): 5921–9. doi:10.1021/bi049444o. PMID 15147175.
- ↑ Hasan SS, Zakharov SD, Chauvet A, Stadnytskyi V, Savikhin S, Cramer WA (Jun 2014). "A map of dielectric heterogeneity in a membrane protein: the hetero-oligomeric cytochrome b6f complex". The Journal of Physical Chemistry B. 118 (24): 6614–25. doi:10.1021/jp501165k. PMC 4067154. PMID 24867491.
- 1 2 Berg JM, Tymoczko JL, Stryer L, Stryer L (2007). Biochemistry. New York: W.H. Freeman. ISBN 978-0-7167-8724-2.
- ↑ Munekage Y, Hashimoto M, Miyake C, Tomizawa K, Endo T, Tasaka M, Shikanai T (Jun 2004). "Cyclic electron flow around photosystem I is essential for photosynthesis". Nature. 429 (6991): 579–82. Bibcode:2004Natur.429..579M. doi:10.1038/nature02598. PMID 15175756. S2CID 4421776.
- ↑ Blankenship RE (2002). Molecular mechanisms of photosynthesis. Oxford ; Malden, MA: Blackwell Science. ISBN 978-0-632-04321-7.
- ↑ Bendall D (1995). "Cyclic photophosphorylation and electron transport". Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1229: 23–38. doi:10.1016/0005-2728(94)00195-B.
- ↑ Baniulis D, Hasan SS, Stofleth JT, Cramer WA (Dec 2013). "Mechanism of enhanced superoxide production in the cytochrome b(6)f complex of oxygenic photosynthesis". Biochemistry. 52 (50): 8975–83. doi:10.1021/bi4013534. PMC 4037229. PMID 24298890.
- ↑ Hasan SS, Proctor EA, Yamashita E, Dokholyan NV, Cramer WA (Oct 2014). "Traffic within the cytochrome b6f lipoprotein complex: gating of the quinone portal". Biophysical Journal. 107 (7): 1620–8. Bibcode:2014BpJ...107.1620H. doi:10.1016/j.bpj.2014.08.003. PMC 4190601. PMID 25296314.
- ↑ Cramer WA, Soriano GM, Ponomarev M, Huang D, Zhang H, Martinez SE, Smith JL (Jun 1996). "Some New Structural Aspects and Old Controversies Concerning the Cytochrome b6f Complex of Oxygenic Photosynthesis". Annual Review of Plant Physiology and Plant Molecular Biology. 47: 477–508. doi:10.1146/annurev.arplant.47.1.477. PMID 15012298.
- 1 2 Cramer WA, Zhang H, Yan J, Kurisu G, Smith JL (2006). "Transmembrane traffic in the cytochrome b6f complex". Annual Review of Biochemistry. 75: 769–90. doi:10.1146/annurev.biochem.75.103004.142756. PMID 16756511.
- ↑ Malone LA, Qian P, Mayneord GE, Hitchcock A, Farmer DA, Thompson RF, et al. (November 2019). "Cryo-EM Structure of the Spinach Cytochrome B 6 F Complex at 3.6 Å Resolution" (PDF). Nature. 575 (7783): 535–539. doi:10.1038/s41586-019-1746-6. PMID 31723268. S2CID 207987984.
- 1 2 Joliot P, Joliot A (Jul 2002). "Cyclic electron transfer in plant leaf". Proceedings of the National Academy of Sciences of the United States of America. 99 (15): 10209–14. Bibcode:2002PNAS...9910209J. doi:10.1073/pnas.102306999. PMC 126649. PMID 12119384.
- ↑ Cramer WA, Yan J, Zhang H, Kurisu G, Smith JL (2005). "Structure of the cytochrome b6f complex: new prosthetic groups, Q-space, and the 'hors d'oeuvres hypothesis' for assembly of the complex". Photosynthesis Research. 85 (1): 133–43. Bibcode:2005PhoRe..85..133C. doi:10.1007/s11120-004-2149-5. PMID 15977064. S2CID 20731696.
Further reading
- Sarewicz, M; Pintscher, S; Pietras, R; Borek, A; Bujnowicz, Ł; Hanke, G; Cramer, WA; Finazzi, G; Osyczka, A (24 February 2021). "Catalytic Reactions and Energy Conservation in the Cytochrome bc(1) and b(6)f Complexes of Energy-Transducing Membranes". Chemical Reviews. 121 (4): 2020–2108. doi:10.1021/acs.chemrev.0c00712. PMC 7908018. PMID 33464892.
External links
- Structure-Function Studies of the Cytochrome b6f Complex - Current research on cytochrome b6f in William Cramer's Lab at Purdue University, USA
- UMich Orientation of Proteins in Membranes families/superfamily-3 - Calculated positions of b6f and related complexes in membranes
- Cytochrome+b6f+Complex at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Plastoquinol-plastocyanin+reductase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)