Portal maintenance status: (May 2019)
|
The Minerals Portal
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid substance with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.
The geological definition of mineral normally excludes compounds that occur only in living organisms. However, some minerals are often biogenic (such as calcite) or organic compounds in the sense of chemistry (such as mellite). Moreover, living organisms often synthesize inorganic minerals (such as hydroxylapatite) that also occur in rocks.
The concept of mineral is distinct from rock, which is any bulk solid geologic material that is relatively homogeneous at a large enough scale. A rock may consist of one type of mineral or may be an aggregate of two or more different types of minerals, spacially segregated into distinct phases.
Some natural solid substances without a definite crystalline structure, such as opal or obsidian, are more properly called mineraloids. If a chemical compound occurs naturally with different crystal structures, each structure is considered a different mineral species. Thus, for example, quartz and stishovite are two different minerals consisting of the same compound, silicon dioxide. (Full article...)
Mineralogy is a subject of geology specializing in the scientific study of the chemistry, crystal structure, and physical (including optical) properties of minerals and mineralized artifacts. Specific studies within mineralogy include the processes of mineral origin and formation, classification of minerals, their geographical distribution, as well as their utilization. (Full article...)
Selected articles
![Image 1Garnets ( /ˈɡɑːrnɪt/) are a group of silicate minerals that have been used since the Bronze Age as gemstones and abrasives.All species of garnets possess similar physical properties and crystal forms, but differ in chemical composition. The different species are pyrope, almandine, spessartine, grossular (varieties of which are hessonite or cinnamon-stone and tsavorite), uvarovite and andradite. The garnets make up two solid solution series: pyrope-almandine-spessartine (pyralspite), with the composition range [Mg,Fe,Mn]3Al2(SiO4)3; and uvarovite-grossular-andradite (ugrandite), with the composition range Ca3[Cr,Al,Fe]2(SiO4)3. (Full article...)](../I/Blank.png.webp) Image 1
Image 1
Garnets ( /ˈɡɑːrnɪt/) are a group of silicate minerals that have been used since the Bronze Age as gemstones and abrasives.
All species of garnets possess similar physical properties and crystal forms, but differ in chemical composition. The different species are pyrope, almandine, spessartine, grossular (varieties of which are hessonite or cinnamon-stone and tsavorite), uvarovite and andradite. The garnets make up two solid solution series: pyrope-almandine-spessartine (pyralspite), with the composition range [Mg,Fe,Mn]3Al2(SiO4)3; and uvarovite-grossular-andradite (ugrandite), with the composition range Ca3[Cr,Al,Fe]2(SiO4)3. (Full article...) Image 2
Image 2 A rock containing three crystals of pyrite (FeS2). The crystal structure of pyrite is primitive cubic, and this is reflected in the cubic symmetry of its natural crystal facets.
A rock containing three crystals of pyrite (FeS2). The crystal structure of pyrite is primitive cubic, and this is reflected in the cubic symmetry of its natural crystal facets.
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties of these crystals:- Primitive cubic (abbreviated cP and alternatively called simple cubic)
- Body-centered cubic (abbreviated cI or bcc)
- Face-centered cubic (abbreviated cF or fcc)
Note: the term fcc is often used in synonym for the cubic close-packed or ccp structure occurring in metals. However, fcc stands for a face-centered-cubic Bravais lattice, which is not necessarily close-packed when a motif is set onto the lattice points. E.g. the diamond and the zincblende lattices are fcc but not close-packed.
Each is subdivided into other variants listed below. Although the unit cells in these crystals are conventionally taken to be cubes, the primitive unit cells often are not. (Full article...) Image 3
Image 3 A naturally-cut diamond crystal
A naturally-cut diamond crystal
Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Another solid form of carbon known as graphite is the chemically stable form of carbon at room temperature and pressure, but diamond is metastable and converts to it at a negligible rate under those conditions. Diamond has the highest hardness and thermal conductivity of any natural material, properties that are used in major industrial applications such as cutting and polishing tools. They are also the reason that diamond anvil cells can subject materials to pressures found deep in the Earth.
Because the arrangement of atoms in diamond is extremely rigid, few types of impurity can contaminate it (two exceptions are boron and nitrogen). Small numbers of defects or impurities (about one per million of lattice atoms) color diamond blue (boron), yellow (nitrogen), brown (defects), green (radiation exposure), purple, pink, orange, or red. Diamond also has a very high refractive index and a relatively high optical dispersion.
Most natural diamonds have ages between 1 billion and 3.5 billion years. Most were formed at depths between 150 and 250 kilometres (93 and 155 mi) in the Earth's mantle, although a few have come from as deep as 800 kilometres (500 mi). Under high pressure and temperature, carbon-containing fluids dissolved various minerals and replaced them with diamonds. Much more recently (hundreds to tens of million years ago), they were carried to the surface in volcanic eruptions and deposited in igneous rocks known as kimberlites and lamproites.
Synthetic diamonds can be grown from high-purity carbon under high pressures and temperatures or from hydrocarbon gases by chemical vapor deposition (CVD). Imitation diamonds can also be made out of materials such as cubic zirconia and silicon carbide. Natural, synthetic, and imitation diamonds are most commonly distinguished using optical techniques or thermal conductivity measurements. (Full article...) Image 4
Image 4 Graphite specimen
Graphite specimen
Graphite (/ˈɡræfaɪt/) is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on a large scale (1.3 million metric tons per year in 2022) for uses in pencils, lubricants, and electrodes. Under high pressures and temperatures it converts to diamond. It is a good (but not excellent) conductor of both heat and electricity. (Full article...) Image 5
Image 5
Rutile is an oxide mineral composed of titanium dioxide (TiO2), the most common natural form of TiO2. Rarer polymorphs of TiO2 are known, including anatase, akaogiite, and brookite.
Rutile has one of the highest refractive indices at visible wavelengths of any known crystal and also exhibits a particularly large birefringence and high dispersion. Owing to these properties, it is useful for the manufacture of certain optical elements, especially polarization optics, for longer visible and infrared wavelengths up to about 4.5 micrometres. Natural rutile may contain up to 10% iron and significant amounts of niobium and tantalum.
Rutile derives its name from the Latin rutilus ('red'), in reference to the deep red color observed in some specimens when viewed by transmitted light. Rutile was first described in 1803 by Abraham Gottlob Werner using specimens obtained in Horcajuelo de la Sierra, Madrid (Spain), which is consequently the type locality. (Full article...) Image 6
Image 6
Gypsum is a soft sulfate mineral composed of calcium sulfate dihydrate, with the chemical formula CaSO4·2H2O. It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, drywall and blackboard or sidewalk chalk. Gypsum also crystallizes as translucent crystals of selenite. It forms as an evaporite mineral and as a hydration product of anhydrite. The Mohs scale of mineral hardness defines gypsum as hardness value 2 based on scratch hardness comparison.
Fine-grained white or lightly tinted forms of gypsum known as alabaster have been used for sculpture by many cultures including Ancient Egypt, Mesopotamia, Ancient Rome, the Byzantine Empire, and the Nottingham alabasters of Medieval England. (Full article...) Image 7
Image 7
Amethyst is a violet variety of quartz. The name comes from the Koine Greek αμέθυστος amethystos from α- a-, "not" and μεθύσκω (Ancient Greek) methysko / μεθώ metho (Modern Greek), "intoxicate", a reference to the belief that the stone protected its owner from drunkenness. Ancient Greeks wore amethyst and carved drinking vessels from it in the belief that it would prevent intoxication.
Amethyst, a semiprecious stone, is often used in jewelry. (Full article...) Image 8
Image 8 Malachite from the Democratic Republic of the Congo
Malachite from the Democratic Republic of the Congo
Malachite is a copper carbonate hydroxide mineral, with the formula Cu2CO3(OH)2. This opaque, green-banded mineral crystallizes in the monoclinic crystal system, and most often forms botryoidal, fibrous, or stalagmitic masses, in fractures and deep, underground spaces, where the water table and hydrothermal fluids provide the means for chemical precipitation. Individual crystals are rare, but occur as slender to acicular prisms. Pseudomorphs after more tabular or blocky azurite crystals also occur. (Full article...) Image 9
Image 9
Cinnabar (/ˈsɪnəˌbɑːr/; from Ancient Greek κιννάβαρι (kinnábari)), or cinnabarite (/ˌsɪnəˈbɑːraɪt/), is the bright scarlet to brick-red form of mercury(II) sulfide (HgS). It is the most common source ore for refining elemental mercury and is the historic source for the brilliant red or scarlet pigment termed vermilion and associated red mercury pigments.
Cinnabar generally occurs as a vein-filling mineral associated with recent volcanic activity and alkaline hot springs. The mineral resembles quartz in symmetry and in its exhibiting birefringence. Cinnabar has a mean refractive index near 3.2, a hardness between 2.0 and 2.5, and a specific gravity of approximately 8.1. The color and properties derive from a structure that is a hexagonal crystalline lattice belonging to the trigonal crystal system, crystals that sometimes exhibit twinning.
Cinnabar has been used for its color since antiquity in the Near East, including as a rouge-type cosmetic, in the New World since the Olmec culture, and in China since as early as the Yangshao culture, where it was used in coloring stoneware.
Associated modern precautions for use and handling of cinnabar arise from the toxicity of the mercury component, which was recognized as early as ancient Rome. (Full article...) Image 10
Image 10_-_Kopalnia_soli_Wieliczka%252C_Polska.jpg.webp) Halite from the Wieliczka salt mine, Małopolskie, Poland
Halite from the Wieliczka salt mine, Małopolskie, Poland
Halite (/ˈhælaɪt, ˈheɪlaɪt/), commonly known as rock salt, is a type of salt, the mineral (natural) form of sodium chloride (NaCl). Halite forms isometric crystals. The mineral is typically colorless or white, but may also be light blue, dark blue, purple, pink, red, orange, yellow or gray depending on inclusion of other materials, impurities, and structural or isotopic abnormalities in the crystals. It commonly occurs with other evaporite deposit minerals such as several of the sulfates, halides, and borates. The name halite is derived from the Ancient Greek word for "salt", ἅλς (háls). (Full article...) Image 11
Image 11 Quartz crystal cluster from Brazil
Quartz crystal cluster from Brazil
Quartz is a hard, crystalline mineral composed of silica (silicon dioxide). The atoms are linked in a continuous framework of SiO4 silicon–oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall chemical formula of SiO2. Quartz is the second most abundant mineral in Earth's continental crust, behind feldspar.
Quartz exists in two forms, the normal α-quartz and the high-temperature β-quartz, both of which are chiral. The transformation from α-quartz to β-quartz takes place abruptly at 573 °C (846 K; 1,063 °F). Since the transformation is accompanied by a significant change in volume, it can easily induce microfracturing of ceramics or rocks passing through this temperature threshold.
There are many different varieties of quartz, several of which are classified as gemstones. Since antiquity, varieties of quartz have been the most commonly used minerals in the making of jewelry and hardstone carvings, especially in Eurasia.
Quartz is the mineral defining the value of 7 on the Mohs scale of hardness, a qualitative scratch method for determining the hardness of a material to abrasion. (Full article...) Image 12
Image 12 A lustrous crystal of zircon perched on a tan matrix of calcite from the Gilgit District of Pakistan
A lustrous crystal of zircon perched on a tan matrix of calcite from the Gilgit District of Pakistan
Zircon (/ˈzɜːrkɒn, -kən/) is a mineral belonging to the group of nesosilicates and is a source of the metal zirconium. Its chemical name is zirconium(IV) silicate, and its corresponding chemical formula is ZrSiO4. An empirical formula showing some of the range of substitution in zircon is (Zr1–y, REEy)(SiO4)1–x(OH)4x–y. Zircon precipitates from silicate melts and has relatively high concentrations of high field strength incompatible elements. For example, hafnium is almost always present in quantities ranging from 1 to 4%. The crystal structure of zircon is tetragonal crystal system. The natural color of zircon varies between colorless, yellow-golden, red, brown, blue, and green.
The name derives from the Persian zargun, meaning "gold-hued". This word is changed into "jargoon", a term applied to light-colored zircons. The English word "zircon" is derived from Zirkon, which is the German adaptation of this word. Yellow, orange, and red zircon is also known as "hyacinth", from the flower hyacinthus, whose name is of Ancient Greek origin. (Full article...) Image 13
Image 13
Asbestos (/æsˈbɛstəs, æzˈ-, -tɒs/ ass-BEST-əs, az-, -oss) is a naturally occurring fibrous silicate mineral. There are six types, all of which are composed of long and thin fibrous crystals, each fibre (particulate with length substantially greater than width) being composed of many microscopic "fibrils" that can be released into the atmosphere by abrasion and other processes. Inhalation of asbestos fibres can lead to various dangerous lung conditions, including mesothelioma, asbestosis, and lung cancer. As a result of these health effects, asbestos is considered a serious health and safety hazard.
Archaeological studies have found evidence of asbestos being used as far back as the Stone Age to strengthen ceramic pots, but large-scale mining began at the end of the 19th century when manufacturers and builders began using asbestos for its desirable physical properties. Asbestos is an excellent thermal and electrical insulator, and is highly fire resistant, so for much of the 20th century, it was very commonly used across the world as a building material, until its adverse effects on human health were more widely acknowledged in the 1970s. Many buildings constructed before the 1980s contain asbestos.
The use of asbestos for construction and fireproofing has been made illegal in many countries. Despite this, at least 100,000 people are thought to die each year from diseases related to asbestos exposure. In part, this is because many older buildings still contain asbestos; in addition, the consequences of exposure can take decades to arise. The latency period (from exposure to the diagnosis of negative health effects) is typically 20 years. The most common diseases associated with chronic asbestos exposure are asbestosis (scarring of the lungs due to asbestos inhalation) and mesothelioma (a type of cancer).
Many developing countries still support the use of asbestos as a building material, and mining of asbestos is ongoing, with the top producer, Russia, having an estimated production of 790,000 tonnes in 2020. (Full article...) Image 14
Image 14 Mineralogy applies principles of chemistry, geology, physics and materials science to the study of minerals
Mineralogy applies principles of chemistry, geology, physics and materials science to the study of minerals
Mineralogy is a subject of geology specializing in the scientific study of the chemistry, crystal structure, and physical (including optical) properties of minerals and mineralized artifacts. Specific studies within mineralogy include the processes of mineral origin and formation, classification of minerals, their geographical distribution, as well as their utilization. (Full article...) Image 15
Image 15
Apatite is a group of phosphate minerals, usually hydroxyapatite, fluorapatite and chlorapatite, with high concentrations of OH−, F− and Cl− ion, respectively, in the crystal. The formula of the admixture of the three most common endmembers is written as Ca10(PO4)6(OH,F,Cl)2, and the crystal unit cell formulae of the individual minerals are written as Ca10(PO4)6(OH)2, Ca10(PO4)6F2 and Ca10(PO4)6Cl2.
The mineral was named apatite by the German geologist Abraham Gottlob Werner in 1786, although the specific mineral he had described was reclassified as fluorapatite in 1860 by the German mineralogist Karl Friedrich August Rammelsberg. Apatite is often mistaken for other minerals. This tendency is reflected in the mineral's name, which is derived from the Greek word ἀπατάω (apatáō), which means to deceive. (Full article...) Image 16
Image 16 Deep green isolated fluorite crystal resembling a truncated octahedron, set upon a micaceous matrix, from Erongo Mountain, Erongo Region, Namibia (overall size: 50 mm × 27 mm, crystal size: 19 mm wide, 30 g)
Deep green isolated fluorite crystal resembling a truncated octahedron, set upon a micaceous matrix, from Erongo Mountain, Erongo Region, Namibia (overall size: 50 mm × 27 mm, crystal size: 19 mm wide, 30 g)
Fluorite (also called fluorspar) is the mineral form of calcium fluoride, CaF2. It belongs to the halide minerals. It crystallizes in isometric cubic habit, although octahedral and more complex isometric forms are not uncommon.
The Mohs scale of mineral hardness, based on scratch hardness comparison, defines value 4 as fluorite.
Pure fluorite is colourless and transparent, both in visible and ultraviolet light, but impurities usually make it a colorful mineral and the stone has ornamental and lapidary uses. Industrially, fluorite is used as a flux for smelting, and in the production of certain glasses and enamels. The purest grades of fluorite are a source of fluoride for hydrofluoric acid manufacture, which is the intermediate source of most fluorine-containing fine chemicals. Optically clear transparent fluorite lenses have low dispersion, so lenses made from it exhibit less chromatic aberration, making them valuable in microscopes and telescopes. Fluorite optics are also usable in the far-ultraviolet and mid-infrared ranges, where conventional glasses are too opaque for use. (Full article...) Image 17
Image 17.jpg.webp)
Micas (/ˈmaɪkəz/ MY-kəz) are a group of silicate minerals whose outstanding physical characteristic is that individual mica crystals can easily be split into extremely thin elastic plates. This characteristic is described as perfect basal cleavage. Mica is common in igneous and metamorphic rock and is occasionally found as small flakes in sedimentary rock. It is particularly prominent in many granites, pegmatites, and schists, and "books" (large individual crystals) of mica several feet across have been found in some pegmatites.
Micas are used in products such as drywalls, paints, fillers, especially in parts for automobiles, roofing and shingles, as well as in electronics. The mineral is used in cosmetics and food to add "shimmer" or "frost." (Full article...) Image 18
Image 18 A crystalline solid: atomic resolution image of strontium titanate. Brighter spots are columns of strontium atoms and darker ones are titanium-oxygen columns.
A crystalline solid: atomic resolution image of strontium titanate. Brighter spots are columns of strontium atoms and darker ones are titanium-oxygen columns.
Crystallography is the experimental science of determining the arrangement of atoms in crystalline solids. Crystallography is a fundamental subject in the fields of materials science and solid-state physics (condensed matter physics). The word crystallography is derived from the Ancient Greek word κρύσταλλος (krústallos; "clear ice, rock-crystal"), with its meaning extending to all solids with some degree of transparency, and γράφειν (gráphein; "to write"). In July 2012, the United Nations recognised the importance of the science of crystallography by proclaiming that 2014 would be the International Year of Crystallography.
Before the development of X-ray diffraction crystallography (see below), the study of crystals was based on physical measurements of their geometry using a goniometer. This involved measuring the angles of crystal faces relative to each other and to theoretical reference axes (crystallographic axes), and establishing the symmetry of the crystal in question. The position in 3D space of each crystal face is plotted on a stereographic net such as a Wulff net or Lambert net. The pole to each face is plotted on the net. Each point is labelled with its Miller index. The final plot allows the symmetry of the crystal to be established.
Crystallographic methods depend mainly on analysis of the diffraction patterns of a sample targeted by a beam of some type. X-rays are most commonly used; other beams used include electrons or neutrons. Crystallographers often explicitly state the type of beam used, as in the terms X-ray crystallography, neutron diffraction and electron diffraction. These three types of radiation interact with the specimen in different ways.- X-rays interact with the spatial distribution of electrons in the sample.
- Neutrons are scattered by the atomic nuclei through the strong nuclear forces, but in addition, the magnetic moment of neutrons is non-zero. They are therefore also scattered by magnetic fields. When neutrons are scattered from hydrogen-containing materials, they produce diffraction patterns with high noise levels. However, the material can sometimes be treated to substitute deuterium for hydrogen. Because of these different forms of interaction, the three types of radiation are suitable for different crystallographic studies.
- Electrons are charged particles and therefore interact with the total charge distribution of both the atomic nuclei and the electrons of the sample.
It is hard to focus x-rays or neutrons, but since electrons are charged they can be focused and are used in electron microscope to produce magnified images. There are many ways that transmission electron microscopy and related techniques such as scanning transmission electron microscopy, high-resolution electron microscopy can be used to obtain images with in many cases atomic resolution from which crystallographic information can be obtained. There are also other methods such as low-energy electron diffraction, low-energy electron microscopy and reflection high-energy electron diffraction which can be used to obtain crystallographic information about surfaces. (Full article...) Image 19
Image 19 Crystal structure of table salt (sodium in purple, chlorine in green)
Crystal structure of table salt (sodium in purple, chlorine in green)
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric patterns that repeat along the principal directions of three-dimensional space in matter.
The smallest group of particles in the material that constitutes this repeating pattern is the unit cell of the structure. The unit cell completely reflects the symmetry and structure of the entire crystal, which is built up by repetitive translation of the unit cell along its principal axes. The translation vectors define the nodes of the Bravais lattice.
The lengths of the principal axes, or edges, of the unit cell and the angles between them are the lattice constants, also called lattice parameters or cell parameters. The symmetry properties of the crystal are described by the concept of space groups. All possible symmetric arrangements of particles in three-dimensional space may be described by the 230 space groups.
The crystal structure and symmetry play a critical role in determining many physical properties, such as cleavage, electronic band structure, and optical transparency. (Full article...) Image 20
Image 20
Beryl (/ˈbɛrəl/ BERR-əl) is a mineral composed of beryllium aluminium silicate with the chemical formula Be3Al2Si6O18. Well-known varieties of beryl include emerald and aquamarine. Naturally occurring, hexagonal crystals of beryl can be up to several meters in size, but terminated crystals are relatively rare. Pure beryl is colorless, but it is frequently tinted by impurities; possible colors are green, blue, yellow, pink, and red (the rarest). It is an ore source of beryllium. (Full article...) Image 21
Image 21
Talc, or talcum, is a clay mineral composed of hydrated magnesium silicate, with the chemical formula Mg3Si4O10(OH)2. Talc in powdered form, often combined with corn starch, is used as baby powder. This mineral is used as a thickening agent and lubricant. It is an ingredient in ceramics, paints, and roofing material. It is a main ingredient in many cosmetics. It occurs as foliated to fibrous masses, and in an exceptionally rare crystal form. It has a perfect basal cleavage and an uneven flat fracture, and it is foliated with a two-dimensional platy form.
The Mohs scale of mineral hardness, based on scratch hardness comparison, defines value 1 as the hardness of talc, the softest mineral. When scraped on a streak plate, talc produces a white streak; though this indicator is of little importance, because most silicate minerals produce a white streak. Talc is translucent to opaque, with colors ranging from whitish grey to green with a vitreous and pearly luster. Talc is not soluble in water, and is slightly soluble in dilute mineral acids.
Soapstone is a metamorphic rock composed predominantly of talc. (Full article...) Image 22
Image 22 A sample of andesite (dark groundmass) with amygdaloidal vesicles filled with zeolite. Diameter of view is 8 cm.
A sample of andesite (dark groundmass) with amygdaloidal vesicles filled with zeolite. Diameter of view is 8 cm.
Andesite (/ˈændəzaɪt/) is a volcanic rock of intermediate composition. In a general sense, it is the intermediate type between silica-poor basalt and silica-rich rhyolite. It is fine-grained (aphanitic) to porphyritic in texture, and is composed predominantly of sodium-rich plagioclase plus pyroxene or hornblende.
Andesite is the extrusive equivalent of plutonic diorite. Characteristic of subduction zones, andesite represents the dominant rock type in island arcs. The average composition of the continental crust is andesitic. Along with basalts, andesites are a component of the Martian crust.
The name andesite is derived from the Andes mountain range, where this rock type is found in abundance. It was first applied by Christian Leopold von Buch in 1826. (Full article...) Image 23
Image 23
Tourmaline (/ˈtʊərməlɪn, -ˌliːn/ TOOR-mə-lin, -leen) is a crystalline silicate mineral group in which boron is compounded with elements such as aluminium, iron, magnesium, sodium, lithium, or potassium. This gemstone comes in a wide variety of colors.
The name is derived from the Sinhalese tōramalli (ටෝරමල්ලි), which refers to the carnelian gemstones. (Full article...) Image 24
Image 24 Green fluorite with prominent cleavage
Green fluorite with prominent cleavage
Cleavage, in mineralogy and materials science, is the tendency of crystalline materials to split along definite crystallographic structural planes. These planes of relative weakness are a result of the regular locations of atoms and ions in the crystal, which create smooth repeating surfaces that are visible both in the microscope and to the naked eye. If bonds in certain directions are weaker than others, the crystal will tend to split along the weakly bonded planes. These flat breaks are termed "cleavage". The classic example of cleavage is mica, which cleaves in a single direction along the basal pinacoid, making the layers seem like pages in a book. In fact, mineralogists often refer to "books of mica".
Diamond and graphite provide examples of cleavage. Each is composed solely of a single element, carbon. In diamond, each carbon atom is bonded to four others in a tetrahedral pattern with short covalent bonds. The planes of weakness (cleavage planes) in a diamond are in four directions, following the faces of the octahedron. In graphite, carbon atoms are contained in layers in a hexagonal pattern where the covalent bonds are shorter (and thus even stronger) than those of diamond. However, each layer is connected to the other with a longer and much weaker van der Waals bond. This gives graphite a single direction of cleavage, parallel to the basal pinacoid. So weak is this bond that it is broken with little force, giving graphite a slippery feel as layers shear apart. As a result, graphite makes an excellent dry lubricant.
While all single crystals will show some tendency to split along atomic planes in their crystal structure, if the differences between one direction or another are not large enough, the mineral will not display cleavage. Corundum, for example, displays no cleavage. (Full article...) Image 25Zeolite is a family of several microporous, crystalline aluminosilicate materials commonly used as commercial adsorbents and catalysts. They mainly consist of silicon, aluminium, oxygen, and have the general formula Mn+
Image 25Zeolite is a family of several microporous, crystalline aluminosilicate materials commonly used as commercial adsorbents and catalysts. They mainly consist of silicon, aluminium, oxygen, and have the general formula Mn+
1/n(AlO
2)−
(SiO
2)
x・yH
2O where Mn+
1/n is either a metal ion or H+. These positive ions can be exchanged for others in a contacting electrolyte solution. H+
exchanged zeolites are particularly useful as solid acid catalysts.
The term was originally coined in 1756 by Swedish mineralogist Axel Fredrik Cronstedt, who observed that rapidly heating a material, believed to have been stilbite, produced large amounts of steam from water that had been adsorbed by the material. Based on this, he called the material zeolite, from the Greek ζέω (zéō), meaning "to boil" and λίθος (líthos), meaning "stone".
Zeolites occur naturally, but are also produced industrially on a large scale. , 253 unique zeolite frameworks have been identified, and over 40 naturally occurring zeolite frameworks are known. Every new zeolite structure that is obtained is examined by the International Zeolite Association Structure Commission (IZA-SC) and receives a three-letter designation. (Full article...)
Selected mineralogist
 Image 1
Image 1 Karl August Lossen
Karl August Lossen
Karl August Lossen (born Kreuznach (Rhineland), 5 January 1841; died Berlin, 24 February 1893) was a German petrologist and geologist.
After finishing his studies at the gymnasium of Kreuznach in 1859 Lossen became a mining engineer; he began by two and a half years of practical work, then studied at the Universities of Berlin and Halle, where he graduated in 1866; in the same year he became assistant geologist of the Prussian national geological survey. He began immediately his well-known petrolographic studies of the Harz Mountains, which lasted till his death. In 1870 he became instructor in petrology at the Berlin mining academy, and at the same time lecturer at the university. In 1873, he was made a member of the newly founded Prussian National Geological Institute, and in 1882 received the title of professor; he was a fellow of the Görres Society from its foundation. In 1886, he became an associate professor in the university. (Full article...) Image 2
Image 2 Karl Franz Anton von Schreibers
Karl Franz Anton von Schreibers
Carl Franz Anton Ritter von Schreibers (15 August 1775 – 21 May 1852) was an Austrian naturalist who was a native of Pressburg, Hungary, Habsburg Empire (today Bratislava, Slovakia).
In 1847, an uncommon iron-nickel-phosphide ((Fe,Ni)3P) mineral was named in his honor by Wilhelm Karl Ritter von Haidinger (1775–1871). The mineral is found in meteorites, and is known today as schreibersite. As a zoologist, he was the first to perform a comprehensive anatomical study of the olm, a cave-dwelling, aquatic amphibian. The plant genus Schreibersia (synonym Augusta, family Rubiaceae) was named in his honor by Johann Baptist Emanuel Pohl. (Full article...) Image 3Wilhelm Hermann Julius Eitel (6 May 1891, Frankfurt am Main – 20 July 1979, United States) was a German-American scientist. (Full article...)
Image 3Wilhelm Hermann Julius Eitel (6 May 1891, Frankfurt am Main – 20 July 1979, United States) was a German-American scientist. (Full article...) Image 4
Image 4 Alexander von Schrenk
Alexander von Schrenk
Alexander Gustav von Schrenk (4 February 1816 – 25 June 1876) was a Baltic German-Russian naturalist born near Tula in what was then the Russian Empire. He was a brother to zoologist Leopold von Schrenck (1826–1894).
From 1834 to 1837, he studied sciences at the University of Dorpat (Tartu), later spending several years as an assistant at the botanical garden in St. Petersburg. He was habilitated for mineralogy at Dorpat, where from 1849 he served as a lecturer. From 1858 he spent the next ten years at his wife's manor in Pühajärve (Heiligensee), Livonia, returning to Dorpat in 1868, where he died several years later. (Full article...) Image 5Henri Hureau de Sénarmont (6 September 1808 – 30 June 1862) was a French mineralogist and physicist.
Image 5Henri Hureau de Sénarmont (6 September 1808 – 30 June 1862) was a French mineralogist and physicist.
He was born in Broué, Eure-et-Loir. From 1822 to 1826, he studied at the École Polytechnique in Paris, then furthered his education at the École des Mines. During the course of his career, he became engineer-in-chief of mines, and professor of mineralogy and director of studies at the École des Mines in Paris. (Full article...)![Image 6Portrait by Oscar Pereira da SilvaJosé Bonifácio de Andrada e Silva (Portuguese pronunciation: [ʒuˈzɛ boniˈfasju dʒi ɐ̃ˈdɾadɐ i ˈsiwvɐ]; 13 June 1763 – 6 April 1838) was a Brazilian statesman, naturalist, mineralist, professor and poet, born in Santos, São Paulo, then part of the Portuguese Empire. He was one of the most important mentors of Brazilian independence, and his actions were decisive for the success of Emperor Pedro I. He supported public education, was an abolitionist and suggested that a new national capital be created in Brazil's underdeveloped interior (effected over a century later as Brasília). His career as naturalist was marked by the discovery of four new minerals. (Full article...)](../I/Blank.png.webp) Image 6
Image 6 Portrait by Oscar Pereira da Silva
Portrait by Oscar Pereira da Silva
José Bonifácio de Andrada e Silva (Portuguese pronunciation: [ʒuˈzɛ boniˈfasju dʒi ɐ̃ˈdɾadɐ i ˈsiwvɐ]; 13 June 1763 – 6 April 1838) was a Brazilian statesman, naturalist, mineralist, professor and poet, born in Santos, São Paulo, then part of the Portuguese Empire.
He was one of the most important mentors of Brazilian independence, and his actions were decisive for the success of Emperor Pedro I. He supported public education, was an abolitionist and suggested that a new national capital be created in Brazil's underdeveloped interior (effected over a century later as Brasília). His career as naturalist was marked by the discovery of four new minerals. (Full article...) Image 7Douglas Saxon Coombs CNZM (23 November 1924 – 23 December 2016) was a New Zealand mineralogist and petrologist. (Full article...)
Image 7Douglas Saxon Coombs CNZM (23 November 1924 – 23 December 2016) was a New Zealand mineralogist and petrologist. (Full article...) Image 8Adolarius Jacob Forster (1739–1806) was a Prussian mineralogist and dealer in display specimen minerals. The Forster family left Yorkshire in 1649 and settled in Prussia. Adolarius Jacob Forster began dealing in mineral specimens around 1766, at the age of 27. He continued in that profession for 40 years and travelled widely. He had premises in London, Paris and St. Petersburg. The Covent Garden, London shop and one in Soho was run by his wife. His brother, Ingham Henry Forster (1725–1782) ran the business in Paris. Auction catalogues for sales in Paris were written by Rome de l'Isle.
Image 8Adolarius Jacob Forster (1739–1806) was a Prussian mineralogist and dealer in display specimen minerals. The Forster family left Yorkshire in 1649 and settled in Prussia. Adolarius Jacob Forster began dealing in mineral specimens around 1766, at the age of 27. He continued in that profession for 40 years and travelled widely. He had premises in London, Paris and St. Petersburg. The Covent Garden, London shop and one in Soho was run by his wife. His brother, Ingham Henry Forster (1725–1782) ran the business in Paris. Auction catalogues for sales in Paris were written by Rome de l'Isle.
He was related to Johann Georg Adam Forster and Johann Reinhold Forster and his sister married the London dealer naturalist George Humphrey at St-Martin-in-the-Fields, London on August 16, 1768. In 1802 Forster sold a collection to the museum of the St Petersburg Mining Institute, under the auspices of the Emperor of All Russia Alexander I. He spent the last ten years of his life in Russia, and died in St. Petersburg in 1806. The dealership was taken over by his nephew John Henry Heuland. (Full article...) Image 9
Image 9 Joan Abella
Joan Abella
Joan Abella i Creus (Sabadell, Barcelona, 1968) is a Catalan gemmologist and mineralogist who discovered abellaite, a mineral that receives this name in his honor (Full article...) Image 10Karl Ludwig Felix Machatschki (22 September 1895 – 17 February 1970) was an Austrian mineralogist.
Image 10Karl Ludwig Felix Machatschki (22 September 1895 – 17 February 1970) was an Austrian mineralogist.
He was born in Arnfels (near Leibnitz) in Styria, Austria. He studied at the University of Graz, obtaining his habilitation in 1925; in 1927 he joined the group of Victor Goldschmidt in Oslo for one year. In 1930 he was appointed as a professor at the University of Tübingen. He changed university twice, first in 1941 to the University of Munich and finally in 1944 to the University of Vienna. (Full article...) Image 11
Image 11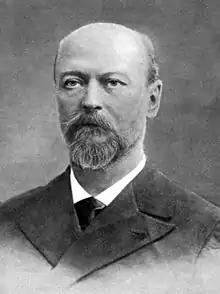
Petru Poni (4 January 1841 – 2 April 1925) was a Moldavian (later Romanian) chemist and mineralogist.
Born into a family of răzeși (free peasants) in Săcărești, Iași County, he attended primary school in Târgu Frumos. In 1852, he enrolled in Academia Mihăileană; among his teachers were August Treboniu Laurian and Simion Bărnuțiu. He entered the University of Paris in 1865, studying chemistry there. He returned home following graduation, teaching physics and chemistry at Iași's National College and at the military high school. In 1878, he became a professor at the University of Iași, at first teaching at the medicine and science faculties, later only in the mineral chemistry department of the latter. He served as Religious Affairs and Education Minister three times: in 1891, 1895-1896 and in 1918. A bitter rival of his was the Conservative Titu Maiorescu, and he was obliged to leave the Liberal cabinet in 1896 after a dispute related to the Romanian Orthodox Church. When not in government, he continued to work in his chemistry laboratory in Iași. (Full article...) Image 12
Image 12 Pencil drawing of Miers by William Rothenstein, 1917
Pencil drawing of Miers by William Rothenstein, 1917
Sir Henry Alexander Miers, FRS (25 May 1858 – 10 December 1942) was a British mineralogist and crystallographer.
Born in Rio de Janeiro, Brazil, he was educated at Eton College and Trinity College, Oxford. He was elected a Fellow of the Royal Society in 1896. (Full article...) Image 13Johann Karl Wilhelm Voigt (20 February 1752 in Allstedt – 2 January 1821 in Ilmenau) was a German mineralogist and mining engineer.
Image 13Johann Karl Wilhelm Voigt (20 February 1752 in Allstedt – 2 January 1821 in Ilmenau) was a German mineralogist and mining engineer.
He initially studied law at the University of Jena, then in 1776 enrolled at the Mining Academy in Freiberg as a pupil of Abraham Gottlob Werner. He later relocated to Weimar, where in 1783 he was named secretary of the Bergwerkskommission (mining commission). During his time spent in Weimar he developed a close friendship with Johann Wolfgang von Goethe — through Voigt, Goethe received an education in mineralogy. From 1789 to 1821, he served as Bergrath (councillor of mines) in Ilmenau. (Full article...) Image 14
Image 14 William Gregor (1761-1817)
William Gregor (1761-1817)
William Gregor (25 December 1761 – 11 June 1817) was an English clergyman and mineralogist who discovered the elemental metal titanium. (Full article...) Image 15
Image 15 Achille Delesse
Achille Delesse
Achille Ernest Oscar Joseph Delesse (3 February 1817 – 24 March 1881) was a French geologist and mineralogist. He is credited for inventing the Delesse principle in stereology. (Full article...) Image 16
Image 16
Ernest-François Mallard (4 February 1833 – 6 July 1894) was a French mineralogist and a member of the French Academy of Sciences. He is also notable for his work with Henri Louis Le Chatelier in combustion as applied to mining safety. (Full article...) Image 17Adolf Schenck (4 April 1857 – 15 September 1936) was a German geographer, mineralogist and botanist who was a native of Siegen. He was a brother to botanist Heinrich Schenck (1860-1927).
Image 17Adolf Schenck (4 April 1857 – 15 September 1936) was a German geographer, mineralogist and botanist who was a native of Siegen. He was a brother to botanist Heinrich Schenck (1860-1927).
Schenck studied at the Universities of Berlin and Bonn, obtaining his doctorate in 1884. From 1884 to 1887 he was a geographer on a mineralogical expedition to German Southwest Africa. The expedition was organized by merchant Adolf Lüderitz (1834-1886) and was under the leadership of Karl Höpfner (1857-1900). Several noted scientists participated in the venture, including Swiss botanist Hans Schinz (1868-1941), who performed botanical investigations in the northern part of German Southwest Africa. In the southern part of the colony, Schenck collected minerals and plants, particularly lichens. Prior to returning to Germany, he visited mines and goldfields that are now located in the present-day nations of South Africa, Botswana and Mozambique. (Full article...) Image 18Matthew Forster Heddle FRSE (28 April 1828 – 19 November 1897) was a Scottish physician and amateur mineralogist active through the 19th century. (Full article...)
Image 18Matthew Forster Heddle FRSE (28 April 1828 – 19 November 1897) was a Scottish physician and amateur mineralogist active through the 19th century. (Full article...) Image 19
Image 19 André Laugier (1770–1832)
André Laugier (1770–1832)
André Laugier (1 August 1770, in Lisieux – 19 April 1832, in Paris) was a French chemist, pharmacist and mineralogist. He was a cousin to famed chemist Antoine François Fourcroy and the father of astronomer Paul Auguste Ernest Laugier (1812–1872).
He received his education in his hometown of Lisieux, and during the French Revolution, was tasked with collecting church bells in Bretagne in order for them to be melted down for the production of cannons. In 1794 he was employed as head of the gunpowder and saltpeter works at the Comite de salut public. In 1797 he received his master's degree in pharmacy, and subsequently taught classes in chemistry and pharmacy at the military training schools in Toulon and Lille. (Full article...) Image 20Hendrik Enno Boeke (12 September 1881, in Wormerveer – 6 December 1918, in Frankfurt am Main) was a Dutch mineralogist and petrographer.
Image 20Hendrik Enno Boeke (12 September 1881, in Wormerveer – 6 December 1918, in Frankfurt am Main) was a Dutch mineralogist and petrographer.
From 1900 he studied chemistry and physics at the University of Amsterdam, where his instructors included Hendrik Willem Bakhuis Roozeboom and Johannes Diderik van der Waals. He then worked as an assistant under Gustav Tammann in Göttingen and to Friedrich Rinne at the Technical University of Hannover. In 1909 he became a lecturer of chemistry at the University of Königsberg, and during the following year, an associate professor of physical-chemical mineralogy and petrology at the University of Leipzig. (Full article...) Image 21
Image 21
Gerardus Troost (March 5, 1776 – August 14, 1850) was a Dutch-American medical doctor, naturalist, mineralogist, and founding member and first president of the Philadelphia Academy of Natural Sciences. (Full article...) Image 22Carlo Perrier (born July 7, 1886, in Turin, † May 22, 1948 in Genoa ) was an Italian mineralogist and chemist who did extensive research on the element technetium. With the discovery of technetium in 1937, he and Emilio Segrè accounted for the last gap in the periodic table. Technetium was the first element produced artificially (hence the name that Segrè and Perrier gave it).
Image 22Carlo Perrier (born July 7, 1886, in Turin, † May 22, 1948 in Genoa ) was an Italian mineralogist and chemist who did extensive research on the element technetium. With the discovery of technetium in 1937, he and Emilio Segrè accounted for the last gap in the periodic table. Technetium was the first element produced artificially (hence the name that Segrè and Perrier gave it).
His parents were named Bertolini. Perrier studied chemistry at the Polytechnic in Turin with a Laureate degree in 1908. From 1911 to 1912 he worked at the Laboratory for Physical Chemistry and Electrochemistry at ETH Zurich with Baur and Treadwell. He then worked as an assistant of Arnaldo Piutti at the University of Naples. There he made friends with Ferruccio Zambonini and involved with mineralogy and the study of radioactivity. He was Zambonini's assistant in Turin and, after a competition, became director of the State Geochemical Laboratory in Rome in 1921. In 1927 he completed his habilitation and after another competition became an associate professor in Messina. In 1929 he relocated to Palermo and in 1939 to the University of Genoa. (Full article...) Image 23
Image 23.jpg.webp) Ludwig Meyn. He was called Dr. Weisheit (Dr. Wisdom) by his friends.
Ludwig Meyn. He was called Dr. Weisheit (Dr. Wisdom) by his friends.
Ludwig Meyn (1 October 1820, Pinneberg − 4 November 1878, Uetersen), was a German agricultural scientist, soil scientist, geologist, journalist and mineralogist. He was the pioneer of oil production. (Full article...) Image 24Friedrich August Frenzel (24 May 1842 – 27 August 1902) was a German mineralogist. He was born in a miner's family in Freiberg, Saxony. In 1861 he was awarded a scholarship which enabled him to study mineralogy at Bergakademie Freiberg. There he attracted the attention of August Breithaupt who asked him to help with organising the mineralogical collections of the academy and with testing mineral samples, and to assist in the professor's mineralogical research. In 1865 Frenzel finished his studies and was awarded the title of a mining inspector. From then on, he worked for 25 years as a chemist in the metallurgical laboratories. He also lectured at the Bergakademie.
Image 24Friedrich August Frenzel (24 May 1842 – 27 August 1902) was a German mineralogist. He was born in a miner's family in Freiberg, Saxony. In 1861 he was awarded a scholarship which enabled him to study mineralogy at Bergakademie Freiberg. There he attracted the attention of August Breithaupt who asked him to help with organising the mineralogical collections of the academy and with testing mineral samples, and to assist in the professor's mineralogical research. In 1865 Frenzel finished his studies and was awarded the title of a mining inspector. From then on, he worked for 25 years as a chemist in the metallurgical laboratories. He also lectured at the Bergakademie.
One of his best known works is the mineralogical encyclopedia for the Kingdom of Saxony (Mineralogisches Lexicon Für Das Königreich Sachsen), which contains descriptions of 723 minerals found in Saxony, information on their physical properties and chemical compositions, and descriptions of the corresponding localities. (Full article...) Image 25
Image 25.png.webp)
Charles Palache (July 18, 1869 – December 5, 1954) was an American mineralogist and crystallographer. In his time, he was one of the most important mineralogists in the United States. (Full article...)
Related portals
Get involved
For editor resources and to collaborate with other editors on improving Wikipedia's Minerals-related articles, see WikiProject Rocks and minerals.
General images

 Image 2Red cinnabar (HgS), a mercury ore, on dolomite. (from Mineral)
Image 2Red cinnabar (HgS), a mercury ore, on dolomite. (from Mineral) Image 3When minerals react, the products will sometimes assume the shape of the reagent; the product mineral is termed a pseudomorph of (or after) the reagent. Illustrated here is a pseudomorph of kaolinite after orthoclase. Here, the pseudomorph preserved the Carlsbad twinning common in orthoclase. (from Mineral)
Image 3When minerals react, the products will sometimes assume the shape of the reagent; the product mineral is termed a pseudomorph of (or after) the reagent. Illustrated here is a pseudomorph of kaolinite after orthoclase. Here, the pseudomorph preserved the Carlsbad twinning common in orthoclase. (from Mineral)
 Image 5Diamond is the hardest natural material, and has a Mohs hardness of 10. (from Mineral)
Image 5Diamond is the hardest natural material, and has a Mohs hardness of 10. (from Mineral) Image 6Epidote often has a distinctive pistachio-green colour. (from Mineral)
Image 6Epidote often has a distinctive pistachio-green colour. (from Mineral)
 Image 8Muscovite, a mineral species in the mica group, within the phyllosilicate subclass (from Mineral)
Image 8Muscovite, a mineral species in the mica group, within the phyllosilicate subclass (from Mineral)
 Image 10Pink cubic halite (NaCl; halide class) crystals on a nahcolite matrix (NaHCO3; a carbonate, and mineral form of sodium bicarbonate, used as baking soda). (from Mineral)
Image 10Pink cubic halite (NaCl; halide class) crystals on a nahcolite matrix (NaHCO3; a carbonate, and mineral form of sodium bicarbonate, used as baking soda). (from Mineral) Image 11Black andradite, an end-member of the orthosilicate garnet group. (from Mineral)
Image 11Black andradite, an end-member of the orthosilicate garnet group. (from Mineral) Image 12Mohs hardness kit, containing one specimen of each mineral on the ten-point hardness scale (from Mohs scale)
Image 12Mohs hardness kit, containing one specimen of each mineral on the ten-point hardness scale (from Mohs scale)
 Image 14Sphalerite crystal partially encased in calcite from the Devonian Milwaukee Formation of Wisconsin (from Mineral)
Image 14Sphalerite crystal partially encased in calcite from the Devonian Milwaukee Formation of Wisconsin (from Mineral) Image 15Perfect basal cleavage as seen in biotite (black), and good cleavage seen in the matrix (pink orthoclase). (from Mineral)
Image 15Perfect basal cleavage as seen in biotite (black), and good cleavage seen in the matrix (pink orthoclase). (from Mineral) Image 16Hübnerite, the manganese-rich end-member of the wolframite series, with minor quartz in the background (from Mineral)
Image 16Hübnerite, the manganese-rich end-member of the wolframite series, with minor quartz in the background (from Mineral) Image 17Schist is a metamorphic rock characterized by an abundance of platy minerals. In this example, the rock has prominent sillimanite porphyroblasts as large as 3 cm (1.2 in). (from Mineral)
Image 17Schist is a metamorphic rock characterized by an abundance of platy minerals. In this example, the rock has prominent sillimanite porphyroblasts as large as 3 cm (1.2 in). (from Mineral)


 Image 21Native gold. Rare specimen of stout crystals growing off of a central stalk, size 3.7 x 1.1 x 0.4 cm, from Venezuela. (from Mineral)
Image 21Native gold. Rare specimen of stout crystals growing off of a central stalk, size 3.7 x 1.1 x 0.4 cm, from Venezuela. (from Mineral) Image 22An example of elbaite, a species of tourmaline, with distinctive colour banding. (from Mineral)
Image 22An example of elbaite, a species of tourmaline, with distinctive colour banding. (from Mineral) Image 23Asbestiform tremolite, part of the amphibole group in the inosilicate subclass (from Mineral)
Image 23Asbestiform tremolite, part of the amphibole group in the inosilicate subclass (from Mineral)
 Image 25Gypsum desert rose (from Mineral)
Image 25Gypsum desert rose (from Mineral)
Did you know ...?

- ... that the uncommon mineral aguilarite (pictured), named for discoverer Ponciano Aguilar, is known from the Americas, Europe, Asia, and Australasia?
- ... that the thallium-rich mineral lorandite from the Allchar deposit is being used to determine the flux of solar neutrinos?
- ... that seamanite is known from only four locations, with three in Michigan and one in Australia?
- ...that jerrygibbsite ((Mn,Zn)9(SiO4)4(OH)2) is a rare mineral of which there are only five known samples in the world?
Subcategories
- Select [►] to view subcategories
Topics
| Overview |  | |
|---|---|---|
| Common minerals | ||
Ore minerals, mineral mixtures and ore deposits | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ores |
| ||||||||
| Deposit types | |||||||||
| Borates | |||||
|---|---|---|---|---|---|
| Carbonates | |||||
| Oxides |
| ||||
| Phosphates | |||||
| Silicates | |||||
| Sulfides | |||||
| Other |
| ||||
| Micas |
|
|---|---|
| Talcs |
|
| Pyrophyllite series |
|
| Kaolinites |
|
| Serpentines |
|
| Corrensites | |
| Smectites and vermiculite family |
|
| Chlorites | |
| Allophanes |
|
| Sepiolites |
|
| Pyrosmalites |
|
| Stilpnomelanes |
|
Structural groups mainly; based on rruff.info/ima, modified | |
| Crystalline | |||||||
|---|---|---|---|---|---|---|---|
| Cryptocrystalline | |||||||
| Amorphous | |||||||
| Miscellaneous | |||||||
| Notable varieties |
| ||||||
Titanium minerals | |||||
|---|---|---|---|---|---|
| Oxide minerals |
| ||||
| Silicate minerals | |||||
| Other | |||||
Gemmological classifications by E. Ya. Kievlenko (1980), updated | |||||||||
| Jewelry stones |
| ||||||||
| Jewelry-Industrial stones |
| ||||||||
| Industrial stones |
| ||||||||
Mineral identification | |
|---|---|
| "Special cases" ("native elements and organic minerals") |
|
|---|---|
| "Sulfides and oxides" |
|
| "Evaporites and similars" |
|
| "Mineral structures with tetrahedral units" (sulfate anion, phosphate anion, silicon, etc.) |
|
Associated Wikimedia
The following Wikimedia Foundation sister projects provide more on this subject:
-
 Commons
Commons
Free media repository -
 Wikibooks
Wikibooks
Free textbooks and manuals -
 Wikidata
Wikidata
Free knowledge base -
 Wikinews
Wikinews
Free-content news -
 Wikiquote
Wikiquote
Collection of quotations -
 Wikisource
Wikisource
Free-content library -
 Wikiversity
Wikiversity
Free learning tools -
 Wiktionary
Wiktionary
Dictionary and thesaurus
References
-
 List of all portals
List of all portals -

-

-

-

-

-

-
-

-

-
 Random portal
Random portal -
 WikiProject Portals
WikiProject Portals
