 | |
| Names | |
|---|---|
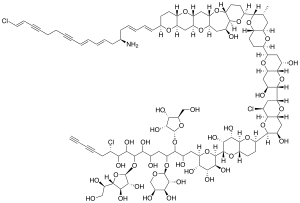
| IUPAC name
77‐Amino‐6,35,90‐trichloro‐17,21:22,26:25,29:30,34:33,37:38,42:41,45:46,50:49,53:54,58:57,62:61,65:64,68:67,71‐tetradecaepoxy‐52‐methyl 14‐(5‐hydroxymethyl‐3,4‐dihydroxy‐2‐oxolanyloxy)‐9‐[5‐(1,2‐dihydroxyethyl)‐3,4‐dihydroxy‐2‐oxolanyloxy]‐13‐(3,4,5‐trihydroxytetrahydro‐2H‐pyran‐2‐yloxy)‐72,74,79,81,89‐nonacontapentene‐1,3,83,87‐tetryne‐7,8,10,11,15,18,19,20,23,24,31,39,43,59‐tetradecol | |
| Other names
PRM1[1] | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| |
| Properties | |
| C107H154Cl3NO44 | |
| Molar mass | 2264.72 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Prymnesin-1 is a chemical with the molecular formula C
107H
154Cl
3NO
44. It is a member of the prymnesins, a class of hemolytic phycotoxins made by the alga Prymnesium parvum.[1][2] It is known to be toxic to fish, causing mass fish deaths around the world, including in Texas and England, or in 2022 in the border region of Germany and Poland (Oder).
Structures
Prymnesin-1 is formed of a large polyether polycyclic core with several conjugate double and triple bonds, chlorine and nitrogen heteroatoms and O-linked sugar moieties including α-D-ribofuranose, α-L-arabinopyranose, and β-D-galactofuranose, unlike the single linked α-L-xylofuranose of prymnesin-2.[1][3] There are three forms of prymnesin known, prymnesin 1 and 2, differing in their glycosylation, and prymnesin B1[4] differing in backbone.
See also
References
- 1 2 3 Igarashi, Tomoji; Satake, Masayuki; Yasumoto, Takeshi (1999). "Structures and Partial Stereochemical Assignments for Prymnesin-1 and Prymnesin-2: Potent Hemolytic and Ichthyotoxic Glycosides Isolated from the Red Tide Alga Prymnesium parvum". J. Am. Chem. Soc. 121 (37): 8499–8511. doi:10.1021/ja991740e.
- ↑ Morohashi, Akio; Satake, Masayuki; Oshima, Yasukatsu; Igarashi, Tomoji; Yasumoto, Takeshi (2001). "Absolute configuration at C14 and C85 in prymnesin-2, a potent hemolytic and ichthyotoxic glycoside isolated from the red tide alga Prymnesium parvum". Chirality. 13 (9): 601–605. doi:10.1002/chir.1184. PMID 11579456.
- ↑ Manning SR, La Claire JW (2010). "Prymnesins: toxic metabolites of the golden alga, Prymnesium parvum carter (Haptophyta)". Marine Drugs. 8 (3): 678–704. doi:10.3390/md8030678. PMC 2857367. PMID 20411121.
- ↑ Rasmussen, Silas Anselm; Meier, Sebastian; Andersen, Nikolaj Gedsted; Blossom, Hannah Eva; Duus, Jens Øllgaard; Nielsen, Kristian Fog; Hansen, Per Juel; Larsen, Thomas Ostenfeld (2016). "Chemodiversity of Ladder-Frame Prymnesin Polyethers in Prymnesium parvum". J. Nat. Prod. 79 (9): 2250–2256. doi:10.1021/acs.jnatprod.6b00345. PMID 27550620.