 | |
| Names | |
|---|---|
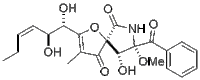
| Preferred IUPAC name
(5S,8S,9R)-8-Benzoyl-2-[(1S,2S,3Z)-1,2-dihydroxyhex-3-en-1-yl]-9-hydroxy-8-methoxy-3-methyl-1-oxa-7-azaspiro[4.4]non-2-ene-4,6-dione | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C22H25NO8 | |
| Molar mass | 431.441 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Pseurotin A is a secondary metabolite of Aspergillus.[1]
Biosynthesis
The gene cluster responsible for the biosynthesis of Pseurotin A was predicted by deletion and overexpression of polyketide synthase nonribosomal peptide synthetase hybrid enzyme gene, PsoA.[2] While it is unknown how the azaspirocyclic core of the pseurotin family of natural products is made, the post polyketide synthase modifications are known. In-vivo and in-vitro studies showed the unique mechanism involved in the modification enzymes PsoC, PsoD, PsoE, and PsoF. PsoD codes for a cytochrome P450, which is responsible for oxidizing the benzyl carbon (C17) to the conjugated ketone. PsoC codes for a methyltransferase, which methylates the tertiary alcohol closest to the benzoyl moiety on C8. PsoE is predicted to be a glutathione S-transferase gene, and isomerizes the C=C bond furthest down the tail from the E stereoisomer to the Z stereoisomer. PsoF is unique in that its gene is encoded separate from the pseurotin gene cluster and is responsible for the epoxidation of the trans double bond that remains in the tail. This epoxide is spontaneously hydrolyzed by either an SN2 mechanism to form pseurotin A, or by an SN2’ mechanism to form pseurotin D.[3]
See also
References
- ↑ Martínez-Luis, S; Cherigo, L; Arnold, E; Spadafora, C; Gerwick, WH; Cubilla-Rios, L (2012). "Antiparasitic and anticancer constituents of the endophytic fungus Aspergillus sp. Strain F1544". Natural Product Communications. 7 (2): 165–8. doi:10.1177/1934578X1200700207. PMID 22474943. S2CID 41167561.
- ↑ Maiya, Shubha; Grundmann, Alexander; Li, Xiang; Li, Shu-Ming; Turner, Geoffrey (2007-09-24). "Identification of a Hybrid PKS/NRPS Required for Pseurotin A Biosynthesis in the Human PathogenAspergillus fumigatus". ChemBioChem. 8 (14): 1736–1743. doi:10.1002/cbic.200700202. ISSN 1439-4227. PMID 17722120. S2CID 9394131.
- ↑ Tsunematsu, Yuta; Fukutomi, Manami; Saruwatari, Takayoshi; Noguchi, Hiroshi; Hotta, Kinya; Tang, Yi; Watanabe, Kenji (2014-06-18). "Elucidation of Pseurotin Biosynthetic Pathway Points to Trans-ActingC-Methyltransferase: Generation of Chemical Diversity". Angewandte Chemie. 126 (32): 8615–8619. doi:10.1002/ange.201404804. ISSN 0044-8249. PMC 4605568. PMID 24939566.
