 | |
| Names | |
|---|---|
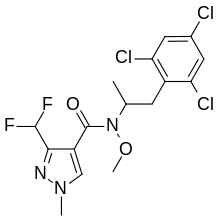
| Preferred IUPAC name
3-(Difluoromethyl)-N-methoxy-1-methyl-N-[1-(2,4,6-trichlorophenyl)-2-propanyl]-1H-pyrazole-4-carboxamide | |
| Other names
SYN545974 | |
| Identifiers | |
3D model (JSmol) |
|
| 20345474 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.252.186 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties[1] | |
| C16H16Cl3F2N3O2 | |
| Molar mass | 426.67 |
| Appearance | Off-white solid |
| Density | g/cm3 |
| Melting point | 113°C |
| 1.5 mg/L (20 °C) | |
| log P | 3.8 |
| Hazards | |
| GHS labelling:[2] | |
  | |
| Warning | |
| H351, H361f, H410 | |
| P203, P273, P280, P318, P391, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Pydiflumetofen is a broad spectrum fungicide used in agriculture to protect crops from fungal diseases. It was first marketed by Syngenta in 2016 using their brand name Miravis. The compound is an amide which combines a pyrazole acid with a substituted phenethylamine to give an inhibitor of succinate dehydrogenase.[3]
History
Inhibition of succinate dehydrogenase, the complex II in the mitochondrial respiration chain, has been known as a fungicidal mechanism of action since the first examples were marketed in the 1960s. The first compound in this class was carboxin, which had a narrow spectrum of useful biological activity, mainly on basidiomycetes and was used as a seed treatment.[4][5] By 2016, at least 17 further examples of this mechanism of action were developed by crop protection companies, with the market leader being boscalid, owing to its broader spectrum of fungal species controlled. However, it lacked full control of important cereal diseases, especially septoria leaf blotch Zymoseptoria tritici.[4]
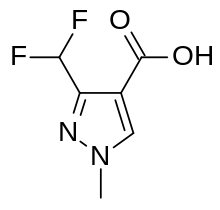
A group of compounds which did control septoria were amides of pyrazole-4-carboxylic acid, with the most successful being derivatives with an N-methyl group and a difluromethyl group in position 3 of the ring. These include penthiopyrad and fluxapyroxad.[6] Research chemists at Syngenta made many analogues of this type[7] in the search for new products and by 2008 had discovered benzovindiflupyr, isopyrazam, sedaxane and pydiflumetofen.[4]
Synthesis
Pydiflumetofen combines 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid with a novel amine derivative which was made from 2,4,6-trichlorobenzaldehyde.[8]
A nitrostyrene is formed in a Henry reaction between the aldehyde and nitroethane. A reduction reaction converts it to a ketone which forms an imine with methoxyamine. This, in turn, is reduced with sodium cyanoborohydride to give the amine required for amide formation with the acid chloride of the pyrazole.[8]
Mechanism of action
Succinate dehydrogenase inhibitors (SDHI) of this type act by binding at the quinone reduction site of the enzyme complex, preventing ubiquinone from doing so. As a consequence, the tricarboxylic acid cycle and electron transport chain cannot function.[9][10][11]
Usage
Pydiflumetofen has fungicidal effects against a wide range of crop pests. These include Alternaria, grey mould (Botrytis cinerea), Cercospora (leaf spot), septoria, powdery mildews (e.g. Uncinula necator), and scab (e.g. Venturia pyrina). As a result, it has potential use in crops including cereals, corn, soybeans, vegetables, peanut, curcubits, potato and fruit.[12][13][14] The compound was introduced in the US in 2018 but estimated usage that year was low at only 4,000 pounds (1,800 kg).[15] The compound is registered for use on peanut and fruits.[14] As of 2023 the compound is also registered in Argentina, Australia, Canada and New Zealand.[16]
Human safety
Pydiflumetofen has low acute toxicity:[11]: 8 the Codex Alimentarius database maintained by the FAO lists the maximum residue limits for it in various food products.[17]
Environmental effects
The compound is very persistent in field conditions and its environmental fate and consequent ecotoxicology have been reviewed.[11]: 11–15 In one laboratory study, the R enantiomer of the compound was shown to be more toxic to Zebrafish, which was interpreted to be owing to its higher potency as an SDHI inhibitor than the S isomer.[18][19]
Resistance management
Fungal populations have the ability to develop resistance to SDHI inhibitors.[20] This potential can be mitigated by careful management. Reports of individual pest species becoming resistant[1] are monitored by manufacturers, regulatory bodies such as the EPA and the Fungicides Resistance Action Committee (FRAC).[21] The risks of resistance developing can be reduced by using a mixture of two or more fungicides which each have activity on relevant pests but with unrelated mechanisms of action. FRAC assigns fungicides into classes so as to facilitate this.[22]
Brands
Pydiflumetofen is the ISO common name[23] for the active ingredient which is formulated into the branded product sold to end-users. Miravis is the brand name for Syngenta's suspension concentrate, which it also calls Adepidyn technology.[14][16]
References
- 1 2 Pesticide Properties Database. "Pydiflumetofen". University of Hertfordshire.
- ↑ "GHS Classification". pubchem.ncbi.nlm.nih.gov. 2023-07-22. Retrieved 2023-07-27.
- ↑ Irie, Makoto (2017). "Pydiflumetofen" (PDF). FAO. Retrieved 2023-07-27.
- 1 2 3 Walter, Harald (2016). "Fungicidal Succinate-Dehydrogenase-Inhibiting Carboxamides". In Lamberth, Clemens; Dinges, Jürgen (eds.). Bioactive Carboxylic Compound Classes: Pharmaceuticals and Agrochemicals. Wiley. pp. 405–425. doi:10.1002/9783527693931.ch31. ISBN 9783527339471.
- ↑ "History of SDHI-fungicides". frac.info. Retrieved 2023-07-26.
- ↑ "Pyrazolecarboxamide fungicides". BCPC. Retrieved 2023-07-27.
- ↑ Walter, Harald; Lamberth, Clemens; Corsi, Camilla (2018). "Synthesis of fungicidally active succinate dehydrogenase inhibitors with novel difluoromethylated heterocyclic acid moieties". Monatshefte für Chemie - Chemical Monthly. 149 (4): 791–799. doi:10.1007/s00706-017-2101-y. S2CID 103548298.
- 1 2 US patent 8258169, Rajan, R; Walter, H & Stierli, D, "Pyrazole-4-N-alkoxycarboxamides as microbiocides", published 2012-09-04, assigned to Syngenta Crop Protection
- ↑ Oyedotun, Kayode S.; Lemire, Bernard D. (2004). "The Quaternary Structure of the Saccharomyces cerevisiae Succinate Dehydrogenase". Journal of Biological Chemistry. 279 (10): 9424–9431. doi:10.1074/jbc.M311876200. PMID 14672929.
- ↑ Avenot, Hervé F.; Michailides, Themis J. (2010). "Progress in understanding molecular mechanisms and evolution of resistance to succinate dehydrogenase inhibiting (SDHI) fungicides in phytopathogenic fungi". Crop Protection. 29 (7): 643–651. doi:10.1016/j.cropro.2010.02.019.
- 1 2 3 Arena, Maria; Auteri, Domenica; Brancato, Alba; et al. (2019). "Peer review of the pesticide risk assessment of the active substance pydiflumetofen". EFSA Journal. 17 (10): e05821. doi:10.2903/j.efsa.2019.5821. PMC 7008818. PMID 32626121.
- ↑ Huang, Xue-Ping; Luo, Jian; Li, Bei-Xing; Song, Yu-fei; Mu, Wei; Liu, Feng (2019). "Bioactivity, physiological characteristics and efficacy of the SDHI fungicide pydiflumetofen against Sclerotinia sclerotiorum". Pesticide Biochemistry and Physiology. 160: 70–78. doi:10.1016/j.pestbp.2019.06.017. PMID 31519259. S2CID 198268036.
- ↑ Duan, Yabing; Xiu, Qian; Li, Haoran; Li, Tao; Wang, Jianxin; Zhou, Mingguo (2019). "Pharmacological Characteristics and Control Efficacy of a Novel SDHI Fungicide Pydiflumetofen Against Sclerotinia sclerotiorum". Plant Disease. 103 (1): 77–82. doi:10.1094/pdis-05-18-0763-re. PMID 30358507. S2CID 53022480.
- 1 2 3 Syngenta US (2021). "Miravis fungicide". Retrieved 2023-07-27.
- ↑ US Geological Survey (2021-10-12). "Estimated Agricultural Use for pydiflumetofen, 2018". Retrieved 2023-07-26.
- 1 2 "ADEPIDYN® technology". syngenta.com. Retrieved 2023-07-27.
- ↑ FAO / WHO. "Pydiflumetofen".
- ↑ Wang, Zhen; Tan, Yuting; Li, Yanhong; Duan, Jinsheng; Wu, Qiqi; Li, Rui; Shi, Haiyan; Wang, Minghua (2022). "Comprehensive study of pydiflumetofen in Danio rerio: Enantioselective insight into the toxic mechanism and fate". Environment International. 167: 107406. doi:10.1016/j.envint.2022.107406. PMID 35850082. S2CID 250463420.
- ↑ Wang, Zhen; Li, Rui; Zhang, Jing; Liu, Shiling; He, Zongzhe; Wang, Minghua (2021). "Evaluation of exploitive potential for higher bioactivity and lower residue risk enantiomer of chiral fungicide pydiflumetofen". Pest Management Science. 77 (7): 3419–3426. doi:10.1002/ps.6389. PMID 33797181. S2CID 232763150.
- ↑ Cosseboom, Scott; Hu, Mengjun (2021). "Identification and Characterization of Fungicide Resistance in Botrytis Populations from Small Fruit Fields in the Mid-Atlantic United States". Plant Disease. 105 (9): 2366–2373. doi:10.1094/pdis-03-20-0487-re. PMID 33719541. S2CID 232231834.
- ↑ "Fungicides Resistance Action Committee website".
- ↑ "Search Fungicides to find FRAC Recommendations". Retrieved 2020-09-04.
- ↑ "Compendium of Pesticide Common Names: Pydiflumetofen". BCPC. Retrieved 2023-07-27.
Further reading
- Jeschke, Peter (2021). "Current Trends in the Design of Fluorine‐Containing Agrochemicals". In Szabó, Kálmán; Selander, Nicklas (eds.). Organofluorine Chemistry. Wiley. pp. 363–395. doi:10.1002/9783527825158.ch11. ISBN 9783527347117. S2CID 234149806.
External links
- PPDB pesticides properties database entry for pydiflumetofen
