 | |
| Names | |
|---|---|
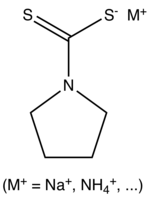
| IUPAC name
Pyrrolidine-1-carbodithioic acid | |
| Other names
Pyrrolidinedithiocarbamate; 1-Pyrrolidinecarbodithioic acid; Pyrrolidine dithiocarbamic acid | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | PDTC |
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H9NS2 | |
| Molar mass | 147.25 g·mol−1 |
| Density | 1.264 g/cm3 |
| Boiling point | 199.7 °C (391.5 °F; 472.8 K) at 760 mm Hg |
| Hazards | |
| Flash point | 74.6 °C (166.3 °F; 347.8 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Pyrrolidine dithiocarbamate (PDTC) are a family of closely related drugs used for a metal chelation, induction of G1 phase cell cycle arrest,[1] and preventing induction of nitric oxide synthase.[2]
Pyrrolidine dithiocarbamate binds zinc such that the resulting complex can enter the cell and inhibit viral RNA-dependent RNA polymerase.[3]
Reactions
Pyrrolidine dithiocarbamate, like other dithiocarbamates, forms coordination complexes with a variety of transition metals. One example is Fe(S2CNC4H8)3.[4]
See also
References
- ↑ Moon, Sung-Kwon; Jung, Sun-Young; Choi, Yung-Hyun; Lee, Young-Choon; Patterson, Cam; Kim, Cheorl-Ho (2004). "PDTC, metal chelating compound, induces G1 phase cell cycle arrest in vascular smooth muscle cells through inducing p21Cip1 expression: Involvement of p38 mitogen activated protein kinase". Journal of Cellular Physiology. 198 (2): 310–23. doi:10.1002/jcp.10728. PMID 14603533.
- ↑ Ammonium pyrrolidinedithiocarbamate at Sigma-Aldrich
- ↑ Lanke, K.; Krenn, B. M.; Melchers, W. J. G.; Seipelt, J.; van Kuppeveld, F. J. M. (1 April 2007). "PDTC inhibits picornavirus polyprotein processing and RNA replication by transporting zinc ions into cells". Journal of General Virology. 88 (4): 1206–1217. doi:10.1099/vir.0.82634-0.
- ↑ Martin, R. L.; Rohde, N. M.; Robertson, G. B.; Taylor, D. (1974). "Structural Characterization of Tris(pyrrolidyldithiocarbamato)iron(IV) Perchlorate. Iron Sulfide (FeS6) Complex of Unusually High Oxidation State". Journal of the American Chemical Society. 96 (11): 3647–3649. doi:10.1021/ja00818a048.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.