 | |
 | |
| Names | |
|---|---|
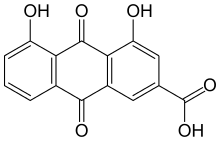
| Preferred IUPAC name
4,5-Dihydroxy-9,10-dioxo-9,10-dihydroanthracene-2-carboxylic acid | |
| Other names | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.006.839 |
| EC Number |
|
| KEGG | |
| MeSH | Rhein |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H8O6 | |
| Molar mass | 284.22 g/mol |
| Appearance | Orange crystals[1] |
| Density | 1.687 g/cm3 |
| Melting point | 350 to 352 °C (662 to 666 °F; 623 to 625 K)[1] |
| Boiling point | 597.8 °C (1,108.0 °F; 870.9 K) at 760 mmHg |
| Insoluble in water | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Irritant |
| GHS labelling: | |
 | |
| Warning | |
| H315, H319, H335 | |
| Flash point | 329.4 °C (624.9 °F; 602.5 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Rhein, also known as cassic acid, is a substance in the anthraquinone group obtained from rhubarb.[2] Like all such substances, rhein is a cathartic, which is commonly found as a glycoside such as rhein-8-glucoside or glucorhein.[2] Rhein was first isolated in 1895.[3] It is found in rhubarb species like Rheum undulatum[4] and Rheum palmatum[5] as well as in Cassia reticulata.[6]
Originally the rhubarb plant which contains rhein was used as a laxative. It was believed that rhein along with other anthraquinone glycosides imparted this activity.[2]
Rhein has been reevaluated as an antibacterial agent against Staphylococcus aureus in 2008.[7] Synergy or partial synergy has been demonstrated between rhein and the antibiotics oxacillin and ampicillin.[8]
Rhein has been shown to inhibit the fat mass and obesity-associated protein, an enzyme responsible for removing the methylation from N6-methyladenosine in nucleic acids.[9][10]
The pharmacokinetics of rhein have not been intensively studied in humans, but at least one study in healthy male volunteers found that rhein was better absorbed from oral administration of rhubarb than from a retention enema.[11] Rhein (at an oral dose of 50 mg twice per day) was shown to be safe when administered for five days to elderly patients with chronic congestive heart failure.[12]
See also
References
- 1 2 Cassic acid on www.naturestandard.com Archived 2011-07-14 at the Wayback Machine
- 1 2 3 Pharmacognosy of Rhubarb | Chemical Constituents
- ↑ Hesse O (1895). "The Chemistry of rhubarb (EN)". Pharmaceutical Journal and Transactions. 1: 325.
- ↑ Lee JH, Kim JM, Kim CS (2003). "Pharmacokinetic analysis of rhein in Rheum undulatum L.". J Ethnopharmacol. 84 (1): 5–9. doi:10.1016/S0378-8741(02)00222-2. PMID 12499069.
- ↑ Hoerhammer L, Wagner H, Koehler I (1959). "Neue Untersuchungen über die Inhaltsstoffe von Rheum palmatum L. 1. Mitteilung: Zur Analytik des Rheins" [New investigations on the components of Rheum palmatum L. Part 1: On the analysis of rhein]. Archiv der Pharmazie (in German). 292 (64): 591–601. doi:10.1002/ardp.19592921105. PMID 14402302. S2CID 94169376.
- ↑ Anchel M (1949). "Identification of the antibiotic substance from Cassia reticulata as 4,5-Dihydroxyanthraquinone-2-carboxylic acid" (PDF). J Biol Chem. 177 (1): 169–177. doi:10.1016/S0021-9258(18)57072-1. PMID 18123056.
- ↑ Yu L, Xiang H, Fan J, et al. (2008). "Global transcriptional response of Staphylococcus aureus to rhein, a natural plant product". J Biotechnol. 135 (3): 304–308. doi:10.1016/j.jbiotec.2008.04.010. PMID 18514345.
- ↑ Joung DK, Joung H, Yang DW, et al. (2012). "Synergistic effect of rhein in combination with ampicillin or oxacillin against methicillin-resistant Staphylococcus aureus". Exp Ther Med. 3 (4): 608–612. doi:10.3892/etm.2012.459. PMC 3438619. PMID 22969937.
- ↑ Yu, Jun; Chen, Mengxian; Huang, Haijiao; Zhu, Junda; Song, Huixue; Zhu, Jian; Park, Jaewon; Ji, Sheng-Jian (2017-11-23). "Dynamic m6A modification regulates local translation of mRNA in axons". Nucleic Acids Research. 46 (3): 1412–1423. doi:10.1093/nar/gkx1182. ISSN 0305-1048. PMC 5815124. PMID 29186567.
- ↑ Jia, Guifang; Fu, Ye; Zhao, Xu; Dai, Qing; Zheng, Guanqun; Yang, Ying; Yi, Chengqi; Lindahl, Tomas; Pan, Tao (2011-10-16). "N6-Methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO". Nature Chemical Biology. 7 (12): 885–887. doi:10.1038/nchembio.687. ISSN 1552-4450. PMC 3218240. PMID 22002720.
- ↑ Zhu W, Wang XM, Zhang L, Li XY, Wang BX (2005). "Pharmacokinetic of rhein in healthy male volunteers following oral and retention enema administration of rhubarb extract: a single dose study". Am J Chin Med. 33 (6): 839–850. doi:10.1142/S0192415X05003508. PMID 16355440.
- ↑ La Villa G, Marra F, Laffi G, et al. (1989). "Effects of rhein on renal arachidonic acid metabolism and renal function in patients with congestive heart failure". Eur J Clin Pharmacol. 37 (1): 1–5. doi:10.1007/bf00609415. PMID 2512175. S2CID 6338421.