 | |
| Names | |
|---|---|
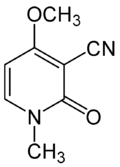
| Preferred IUPAC name
4-Methoxy-1-methyl-2-oxo-1,2-dihydropyridine-3-carbonitrile | |
| Other names
3-cyano-4-methoxy-N-methyl-2-pyridone | |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.601 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H8N2O2 | |
| Molar mass | 164.164 g·mol−1 |
| Melting point | 200 °C (392 °F; 473 K) |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
340 mg/kg[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Ricinine is a toxic alkaloid found in the castor plant.[2] It can serve as a biomarker of ricin poisoning.[3][4] It was first isolated from the castor seeds by Tuson in 1864.[5][6]
Ricinine has insecticidal effects.[7]
It sublimes between 170 and 180 °C at 20 mmHg. It does not form salts, and is precipitated in iodine or mercuric chloride solutions, but not in Mayer's reagent.[5]
It can be hydrolyzed to methanol and ricininic acid by alkali.[5]
See also
References
- ↑ Worbs, Sylvia; Köhler, Kernt; Pauly, Diana; Avondet, Marc-André; Schaer, Martin; Dorner, Martin B.; Dorner, Brigitte G. (24 October 2011). "Ricinus communis Intoxications in Human and Veterinary Medicine—A Summary of Real Cases". Toxins. 3 (10): 1332–1372. doi:10.3390/toxins3101332. PMC 3210461. PMID 22069699.
- ↑ Peng, Jing; Cai, Shuang; Wang, Lin; Zhao, Nan; Zhang, Ting-jian; Chen, Zai-xing; Meng, Fan-hao; Dzeja, Petras (11 March 2014). "A Metabonomic Analysis of Serum from Rats Treated with Ricinine Using Ultra Performance Liquid Chromatography Coupled with Mass Spectrometry". PLOS ONE. 9 (3): e90416. Bibcode:2014PLoSO...990416P. doi:10.1371/journal.pone.0090416. PMC 3949718. PMID 24618672.
- ↑ Hamelin, Elizabeth I.; Johnson, Rudolph C.; Osterloh, John D.; Howard, David J.; Thomas, Jerry D. (November 2012). "Evaluation of Ricinine, a Ricin Biomarker, from a Non-Lethal Castor Bean Ingestion". Journal of Analytical Toxicology. 36 (9): 660–662. doi:10.1093/jat/bks077. PMC 4561852. PMID 23014889.
- ↑ Pittman, C. T.; Guido, J. M.; Hamelin, E. I.; Blake, T. A.; Johnson, R. C. (6 March 2013). "Analysis of a Ricin Biomarker, Ricinine, in 989 Individual Human Urine Samples". Journal of Analytical Toxicology. 37 (4): 237–240. doi:10.1093/jat/bkt010. PMC 4547525. PMID 23471955.
- 1 2 3 Henry, Thomas Anderson (1949). "Ricinine". The Plant Alkaloids (4th ed.). The Blakiston Company. p. 5.
- ↑ Rao, N. V. Subra (Feb 12, 1945). "A Note on the Chemical Composition of Castor Leaves". Proceedings of the Indian Academy of Sciences. A (21): 123–125. doi:10.1007/BF03051280. S2CID 94199266. Retrieved Aug 7, 2021.
- ↑ Wachira, Sabina; Omar, Sabar; Jacob, Julia; Wahome, Martin; Alborn, Hans T; Spring, David R; Masiga, Daniel K; Torto, Baldwyn (2014). "Toxicity of six plant extracts and two pyridone alkaloids from Ricinus communis against the malaria vector Anopheles gambiae". Parasites & Vectors. 7 (1): 312. doi:10.1186/1756-3305-7-312. PMC 4098926. PMID 24996560.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.