SH3 and multiple ankyrin repeat domains protein 1 is a protein that in humans is encoded by the SHANK1 gene.[5][6]
Interactions
SHANK1 has been shown to interact with:
Clinical Significance
SHANK1 is a scaffold protein that plays a critical role in the formation and maintenance of excitatory synapses in the brain. Mutations in the SHANK1 gene have been implicated in a number of neurodevelopmental disorders, including autism spectrum disorder (ASD), schizophrenia, and intellectual disability. In particular, the loss of SHANK1 expression has been linked to ASD, and SHANK1 mutations have been identified in individuals with ASD or other neurodevelopmental disorders. ASD, also known as "autism spectrum disorders", is identified as a group of conditions which cause characteristics in the human brain that lead to impairments. These impairments are such as communication, interest, and socialization issues or patterns of behavioral divergence.
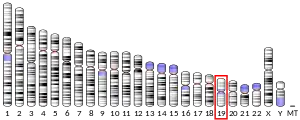
Sato et al 2012 established the significant influence of the mutations and deletions of SHANK1 in males with ASD.[11] This study consisted of 1,158 Canadian individuals and 456 European individuals who all had ASD. SHANK 1 is located is chromosome 19 in humans, while SHANK2 is located on chromosome 11, and SHANK3 on chromosome 22. The locus of SHANK1, in particular, is less studied in relation to ASD than SHANK2 and SHANK3. The objective of the study was to provide more context and analyze the specific protein, SHANK1, as a focal point for male ASD development caused by deletion, microdeletion, and/or mutation. Researchers found that a de novo deletion of the gene was males with high functioning ASD whereas 15,000 males used as a control group, did not have the deletion.
One particular focus of the study took 7 individuals which appeared to have deletions in chromosome 19 involving SHANK1. Of these 7, 4 were males who have high functioning ASD, which also happen to come from multigenerational detection of the inherited mutation and/ or deletion of SHANK1 gene. In that same group, 2 female individuals have SHANK1 deletion, however they did not present with ASD. The last individual of the group was a male who had an unrelated (not inherited) deletion of the SHANK1 gene while presenting ASD. Every individual in this study that presented with ASD was evaluated and diagnosed by an expert clinician using the Autism Diagnostic Observation Schedule (ADOS) and/or the Autism Diagnostic Interview-Revised (ADI-R). Individuals were selected from various hospitals and special clinical centers Throughout Canada and Europe.
A laboratory test was run in order to identify the gene in the individuals using SYBR-Green-based real-time quantitative PCR (qPCR). The test was focused on family 1, the genetic locus in which the SHANK family is found. This allowed for primers to be used in order to establish the presence of any mutations, deletion, or microdeletions of SHANK1. Results indicated that deletion is 63.8 kb, and causes an elimination in exons 1-20 of the SHANK1 gene, as well as deletion in the gene CLEC11A. The CLEC11A gene (MIM 604713) is located on chromosome 2q14.1 and encodes a protein called osteoactivin. Osteoactivin is present in a variety of tissues, including bone, cartilage, and lung. The gene has been found to be a part of several biological processes, including bone remodeling, angiogenesis, and immune response. Mutations in the CLEC11A gene are associated with an increased risk of developing osteoporosis and other bone-related disorders.
In addition to SHANK1 being found to have developing ASD in males with the genetic deletion, the inheritance of the gene was also encountered from the chromosome of the studied individual which originated from the mother. It appeared that the mothers in these cases, carried two copies of the SHANK1 gene. This was not however, the case for presence of ASD in males with mutations of the gene, only deletion.
Researchers found that an unrelated ASD-affected male carrying an independent de novo deletion of SHANK1, supports the interpretation that the SHANK1 CNV segregating in family 1 is in fact the primary etiologic event which leads to the individual presenting ASD. In order to further support this claim, researchers also stated that continuous testing on case reports of multigenerational families would be required. The evidence supports that the mutation, deletion, or microdeletion of the SHANK1 gene is just as influential as SHANK2 and SHANK3 in causing male individuals to present with ASD.
The significance of the findings of the gene mutation extend beyond medical. They also provide understanding as to why there is a diagnosis bias for males versus females with ASD. It also allows for genetic interpretations of the differences in inherited forms of ASD and unrelated genetic mutations. This can have effects on the social aspect of an individual with ASD. Having tested and trusted evidence as to the origin of ASD is crucial as more and more people are diagnosed and the field of resources for these individuals continues to grow.
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000161681 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000038738 - Ensembl, May 2017
- ↑ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- 1 2 Zitzer H, Hönck HH, Bächner D, Richter D, Kreienkamp HJ (January 2000). "Somatostatin receptor interacting protein defines a novel family of multidomain proteins present in human and rodent brain". J Biol Chem. 274 (46): 32997–3001. doi:10.1074/jbc.274.46.32997. PMID 10551867.
- ↑ "Entrez Gene: SHANK1 SH3 and multiple ankyrin repeat domains 1".
- ↑ Park E, Na M, Choi J, Kim S, Lee JR, Yoon J, Park D, Sheng M, Kim E (May 2003). "The Shank family of postsynaptic density proteins interacts with and promotes synaptic accumulation of the beta PIX guanine nucleotide exchange factor for Rac1 and Cdc42". J. Biol. Chem. 278 (21): 19220–9. doi:10.1074/jbc.M301052200. PMID 12626503.
- ↑ Soltau M, Richter D, Kreienkamp HJ (Dec 2002). "The insulin receptor substrate IRSp53 links postsynaptic shank1 to the small G-protein cdc42". Mol. Cell. Neurosci. 21 (4): 575–83. doi:10.1006/mcne.2002.1201. PMID 12504591. S2CID 572407.
- ↑ Okamoto PM, Gamby C, Wells D, Fallon J, Vallee RB (Dec 2001). "Dynamin isoform-specific interaction with the shank/ProSAP scaffolding proteins of the postsynaptic density and actin cytoskeleton". J. Biol. Chem. 276 (51): 48458–65. doi:10.1074/jbc.M104927200. PMC 2715172. PMID 11583995.
- ↑ Böckers TM, Mameza MG, Kreutz MR, Bockmann J, Weise C, Buck F, Richter D, Gundelfinger ED, Kreienkamp HJ (October 2001). "Synaptic scaffolding proteins in rat brain. Ankyrin repeats of the multidomain Shank protein family interact with the cytoskeletal protein alpha-fodrin". J. Biol. Chem. 276 (43): 40104–12. doi:10.1074/jbc.M102454200. PMID 11509555.
- ↑ Sato, Daisuke; Lionel, Anath C.; Leblond, Claire S.; Prasad, Aparna; Pinto, Dalila; Walker, Susan; O'Connor, Irene; Russell, Carolyn; Drmic, Irene E.; Hamdan, Fadi F.; Michaud, Jacques L.; Endris, Volker; Roeth, Ralph; Delorme, Richard; Huguet, Guillaume; Leboyer, Marion; Rastam, Maria; Gillberg, Christopher; Lathrop, Mark; Stavropoulos, Dimitri J.; Anagnostou, Evdokia; Weksberg, Rosanna; Fombonne, Eric; Zwaigenbaum, Lonnie; Fernandez, Bridget A.; Roberts, Wendy; Rappold, Gudrun A.; Marshall, Christian R.; Bourgeron, Thomas; Szatmari, Peter; Scherer, Stephen W. (4 May 2012). "SHANK1 Deletions in Males with Autism Spectrum Disorder". American Journal of Human Genetics. 90 (5): 879–887. doi:10.1016/j.ajhg.2012.03.017. PMC 3376495. PMID 22503632.
Further reading
- Sheng M, Kim E (2000). "The Shank family of scaffold proteins". J. Cell Sci. 113 (11): 1851–6. doi:10.1242/jcs.113.11.1851. PMID 10806096.
- Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschanoff J, Weinberg RJ, Worley PF, Sheng M (1999). "Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin". Neuron. 23 (3): 569–82. doi:10.1016/S0896-6273(00)80809-0. PMID 10433268. S2CID 2209372.
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, Worley PF (1999). "Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins". Neuron. 23 (3): 583–92. doi:10.1016/S0896-6273(00)80810-7. PMID 10433269. S2CID 16429070.
- Tobaben S, Südhof TC, Stahl B (2000). "The G protein-coupled receptor CL1 interacts directly with proteins of the Shank family". J. Biol. Chem. 275 (46): 36204–10. doi:10.1074/jbc.M006448200. PMID 10958799.
- Kreienkamp HJ, Zitzer H, Gundelfinger ED, Richter D, Bockers TM (2000). "The calcium-independent receptor for alpha-latrotoxin from human and rodent brains interacts with members of the ProSAP/SSTRIP/Shank family of multidomain proteins". J. Biol. Chem. 275 (42): 32387–90. doi:10.1074/jbc.C000490200. PMID 10964907.
- Kreienkamp HJ, Zitzer H, Richter D (2001). "Identification of proteins interacting with the rat somatostatin receptor subtype 2". J. Physiol. Paris. 94 (3–4): 193–8. doi:10.1016/S0928-4257(00)00204-7. PMID 11087996. S2CID 8791865.
- Lim S, Sala C, Yoon J, Park S, Kuroda S, Sheng M, Kim E (2001). "Sharpin, a novel postsynaptic density protein that directly interacts with the shank family of proteins". Mol. Cell. Neurosci. 17 (2): 385–97. doi:10.1006/mcne.2000.0940. PMID 11178875. S2CID 45278068.
- Böckers TM, Mameza MG, Kreutz MR, Bockmann J, Weise C, Buck F, Richter D, Gundelfinger ED, Kreienkamp HJ (2001). "Synaptic scaffolding proteins in rat brain. Ankyrin repeats of the multidomain Shank protein family interact with the cytoskeletal protein alpha-fodrin". J. Biol. Chem. 276 (43): 40104–12. doi:10.1074/jbc.M102454200. PMID 11509555.
- Okamoto PM, Gamby C, Wells D, Fallon J, Vallee RB (2002). "Dynamin isoform-specific interaction with the shank/ProSAP scaffolding proteins of the postsynaptic density and actin cytoskeleton". J. Biol. Chem. 276 (51): 48458–65. doi:10.1074/jbc.M104927200. PMC 2715172. PMID 11583995.
- Soltau M, Richter D, Kreienkamp HJ (2003). "The insulin receptor substrate IRSp53 links postsynaptic shank1 to the small G-protein cdc42". Mol. Cell. Neurosci. 21 (4): 575–83. doi:10.1006/mcne.2002.1201. PMID 12504591. S2CID 572407.
- Park E, Na M, Choi J, Kim S, Lee JR, Yoon J, Park D, Sheng M, Kim E (2003). "The Shank family of postsynaptic density proteins interacts with and promotes synaptic accumulation of the beta PIX guanine nucleotide exchange factor for Rac1 and Cdc42". J. Biol. Chem. 278 (21): 19220–9. doi:10.1074/jbc.M301052200. PMID 12626503.
- Daigo Y, Takayama I, Ward SM, Sanders KM, Fujino MA (2004). "Novel human and mouse genes encoding a shank-interacting protein and its upregulation in gastric fundus of W/WV mouse". J. Gastroenterol. Hepatol. 18 (6): 712–8. doi:10.1046/j.1440-1746.2003.03046.x. PMID 12753155. S2CID 38146288.
- Im YJ, Lee JH, Park SH, Park SJ, Rho SH, Kang GB, Kim E, Eom SH (2004). "Crystal structure of the Shank PDZ-ligand complex reveals a class I PDZ interaction and a novel PDZ-PDZ dimerization". J. Biol. Chem. 278 (48): 48099–104. doi:10.1074/jbc.M306919200. PMID 12954649.
- Suzuki T, Li W, Zhang JP, Tian QB, Sakagami H, Usuda N, Usada N, Kondo H, Fujii T, Endo S (2005). "A novel scaffold protein, TANC, possibly a rat homolog of Drosophila rolling pebbles (rols), forms a multiprotein complex with various postsynaptic density proteins". Eur. J. Neurosci. 21 (2): 339–50. doi:10.1111/j.1460-9568.2005.03856.x. PMID 15673434. S2CID 28773407.
- Fieulaine S, Juillan-Binard C, Serero A, Dardel F, Giglione C, Meinnel T, Ferrer JL (2006). "The crystal structure of mitochondrial (Type 1A) peptide deformylase provides clear guidelines for the design of inhibitors specific for the bacterial forms". J. Biol. Chem. 280 (51): 42315–24. doi:10.1074/jbc.M507155200. PMID 16192279.