 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
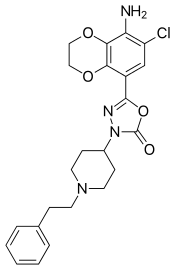
| Formula | C23H25ClN4O4 |
| Molar mass | 456.93 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Capeserod (INN;[1] development code SL65.0155) is a selective 5-HT4 receptor partial agonist with Ki = 0.6 nM and IA = 40–50% (relative to serotonin).[2] It potently enhances cognition, learning, and memory,[2][3][4][5][6][7] and also possesses antidepressant effects.[8] Capeserod was in phase II clinical trials around 2004–2006 for the treatment of memory deficits and dementia but no new information has surfaced since and it appears to have been abandoned.[9][10]
See also
References
- ↑ "WHO Drug Information. Vol. 20, No. 3, 2006. International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 56" (PDF). World Health Organization. p. 208. Archived from the original (PDF) on July 5, 2011. Retrieved 25 April 2016.
- 1 2 Moser PC, Bergis OE, Jegham S, et al. (August 2002). "SL65.0155, a novel 5-hydroxytryptamine(4) receptor partial agonist with potent cognition-enhancing properties" (PDF). The Journal of Pharmacology and Experimental Therapeutics. 302 (2): 731–41. doi:10.1124/jpet.102.034249. PMID 12130738. S2CID 18256582.
- ↑ Spencer JP, Brown JT, Richardson JC, et al. (2004). "Modulation of hippocampal excitability by 5-HT4 receptor agonists persists in a transgenic model of Alzheimer's disease". Neuroscience. 129 (1): 49–54. doi:10.1016/j.neuroscience.2004.06.070. PMID 15489027. S2CID 41720408.
- ↑ Micale V, Leggio GM, Mazzola C, Drago F (November 2006). "Cognitive effects of SL65.0155, a serotonin 5-HT4 receptor partial agonist, in animal models of amnesia". Brain Research. 1121 (1): 207–15. doi:10.1016/j.brainres.2006.08.108. PMID 17011531. S2CID 25494253.
- ↑ Restivo L, Roman F, Dumuis A, Bockaert J, Marchetti E, Ammassari-Teule M (September 2008). "The promnesic effect of G-protein-coupled 5-HT4 receptors activation is mediated by a potentiation of learning-induced spine growth in the mouse hippocampus". Neuropsychopharmacology. 33 (10): 2427–34. doi:10.1038/sj.npp.1301644. PMID 18075492.
- ↑ Marchetti E, Jacquet M, Jeltsch H, et al. (July 2008). "Complete recovery of olfactory associative learning by activation of 5-HT4 receptors after dentate granule cell damage in rats". Neurobiology of Learning and Memory. 90 (1): 185–91. doi:10.1016/j.nlm.2008.03.010. PMID 18485752. S2CID 38967009.
- ↑ Hille C, Bate S, Davis J, Gonzalez MI (December 2008). "5-HT4 receptor agonism in the five-choice serial reaction time task". Behavioural Brain Research. 195 (1): 180–6. doi:10.1016/j.bbr.2008.08.007. PMID 18765258. S2CID 1063310.
- ↑ Tamburella A, Micale V, Navarria A, Drago F (October 2009). "Antidepressant properties of the 5-HT4 receptor partial agonist, SL65.0155: behavioral and neurochemical studies in rats". Progress in Neuro-psychopharmacology & Biological Psychiatry. 33 (7): 1205–10. doi:10.1016/j.pnpbp.2009.07.001. PMID 19596038. S2CID 207408833.
- ↑ Bockaert J, Claeysen S, Compan V, Dumuis A (February 2004). "5-HT4 receptors" (PDF). Current Drug Targets. CNS and Neurological Disorders. 3 (1): 39–51. doi:10.2174/1568007043482615. PMID 14965243. S2CID 19243201.
- ↑ Roth, Bryan L. (2006). The Serotonin Receptors: From Molecular Pharmacology to Human Therapeutics (The Receptors). Totowa, NJ: Humana Press. ISBN 1-58829-568-0.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.