 | |
| Names | |
|---|---|
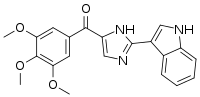
| IUPAC name
[2-(1H-Indol-3-yl)-1H-imidazol-5-yl]-(3,4,5-trimethoxyphenyl)methanone | |
| Other names | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C21H19N3O4 | |
| Molar mass | 377.400 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Sabizabulin is a chemical compound from the group of indole and imidazole derivatives that was first reported in 2012 by Dalton, Li, and Miller.[4] It is being studied as a mitotic inhibitor and chemotherapeutic agent in castration-resistant metastatic prostate cancer[5] and in SARS-CoV-2 (COVID-19) infections.[6]
Properties
Sabizabulin, as an orally available molecule, acts on microtubules, a component of the cytoskeleton. It binds to the colchicine binding site on the beta subunit of tubulin, as well as a novel site on the alpha subunit, and causes both to crosslink, thus depolymerizing microtubules and preventing their polymerization.[7] By preventing mitotic spindle formation, this directly inhibits mitosis of tumor cells and endothelial cells attempting to form new blood vessels to feed them. In parallel, microtubule-mediated trafficking of cellular components (including androgen receptors into the nucleus), thus, a potential anti-androgen agent. The transport of viral particles (including SARS-CoV-2) may also be inhibited. These activities can inhibit viral replication and assembly. Inhibition of tubulin polymerization can also inhibit the release of pro-inflammatory cytokines and disrupt the activities of inflammatory cells.[8]
Sabizabulin is not a substrate of P-glycoprotein (Pgp), an efflux pump that, when overexpressed, can confer resistance to taxanes, a group of widely used cancer therapeutics.
Research
COVID-19 therapy
In a phase III study on the treatment of severe courses of COVID-19,[3][9] sabizabulin reduced mortality by 55% according to the manufacturer.[10] Because of the high efficacy, the test phase was stopped prematurely so that the drug no longer had to be withheld from the placebo control group.[11][12]
References
- ↑ "Substance Name: Sabizabulin". ChemIDplus. Retrieved 1 May 2022.
- ↑ "Sabizabulin for COVID-19". Veru Inc. 14 January 2022. Retrieved 1 May 2022.
- 1 2 "VERU-111 in the Treatment of SARS-Cov-2 Infection by Assessing Its Effect on the Proportion of Patients Who Die on Study". ClinicalTrials.gov (Press release). 13 April 2021. Retrieved 1 May 2022.
- ↑ Li, Chien-Ming; Lu, Yan; Chen, Jianjun; Costello, Terrence A.; Narayanan, Ramesh; Dalton, Mara N.; et al. (4 July 2012). "Orally Bioavailable Tubulin Antagonists for Paclitaxel-Refractory Cancer". Pharmaceutical Research. 29 (11): 3053–3063. doi:10.1007/s11095-012-0814-5. ISSN 0724-8741. PMC 3646298. PMID 22760659.
- ↑ Markowski MC, Tutrone R, Pieczonka C, Barnette KG, Getzenberg RH, Rodriguez D, et al. (April 2022). "A Phase 1b/2 Study of Sabizabulin, a Novel Oral Cytoskeleton Disruptor, in Men With Metastatic Castration-Resistant Prostate Cancer with Progression on an Androgen Receptor Targeting Agent". Clinical Cancer Research. 28 (13): OF1–OF7. doi:10.1158/1078-0432.CCR-22-0162. PMC 9774054. PMID 35416959. S2CID 248128050.
- ↑ rme (13 April 2022). "COVID-19: Krebsmittel Sabizabulin halbiert Sterberate bei schweren Erkrankungen". aerzteblatt.de. Retrieved 14 April 2022.
- ↑ "Sabizabulin for Breast Cancer". Veru Inc. Retrieved 17 May 2022.
- ↑ "Sabizabulin (Code C158517)". NCI Thesaurus. Retrieved 14 April 2022.
- ↑ "Veru Enrolls First Patient in Phase 3 Clinical Trial of Sabizabulin (VERU-111) in High Risk Hospitalized COVID-19 Patients". Veru Inc. 19 May 2021. Retrieved 1 May 2022.
- ↑ "Veru's Novel COVID-19 Drug Candidate Reduces Deaths by 55% in Hospitalized Patients in Interim Analysis of Phase 3 Study; Independent Data Monitoring Committee Halts Study Early for Overwhelming Efficacy". Veru Inc. (Press release). 11 April 2022. Retrieved 30 April 2022.
- ↑ Rabin, Roni (11 April 2022). "New Drug Slashed Deaths Among Patients With Severe Covid, Maker Claims". The New York Times. Retrieved 21 April 2022.
- ↑ "Veru Announces Oral Late-Breaking Presentation of Phase 2 Data of Sabizabulin for the Treatment of Hospitalized Severe COVID-19 Patients at High Risk for Acute Respiratory Distress Syndrome at the 32nd European Congress of Clinical Microbiology & Infectious Diseases" (Press release). Veru Inc. 25 April 2022. Retrieved 30 April 2022 – via GlobeNewswire.
Further reading
- Deng S, Krutilina RI, Wang Q, Lin Z, Parke DN, Playa HC, et al. (February 2020). "An Orally Available Tubulin Inhibitor, VERU-111, Suppresses Triple-Negative Breast Cancer Tumor Growth and Metastasis and Bypasses Taxane Resistance". Molecular Cancer Therapeutics. 19 (2): 348–363. doi:10.1158/1535-7163.MCT-19-0536. PMC 7007836. PMID 31645441.
- Shahnoor S, Khan AM, Habiba U (2023). "Sabizabulin - an unprecedented yet effective drug against COVID-19". Journal of the Pakistan Medical Association. 73 (6): 1363. doi:10.47391/JPMA.7539. PMID 37427659.
External links
- "Sabizabulin". Drug Information Portal. U.S. National Library of Medicine.