 | |
| Names | |
|---|---|
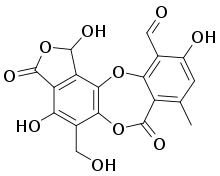
| IUPAC name
1,3-Dihydro-1,4,10-trihydroxy-5-(hydroxymethyl)-8-methyl-3,7-dioxo-7H-isobenzofuro[4,5-b][1,4]benzodioxepin-11-carboxaldehyde | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.558 |
| EC Number |
|
PubChem CID |
|
| |
| |
| Properties | |
| C18H12O10 | |
| Molar mass | 388.284 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Salazinic acid is a depsidone with a lactone ring. It is found in some lichens, and is especially prevalent in Parmotrema and Bulbothrix, where its presence or absence is often used to help classify species in those genera.
History
In 1897, Friedrich Wilhelm Zopf named the chemical he originally isolated from the African species Stereocaulon salazinum as salazinic acid.[1] Later studies showed that the compound he named was actually norstictic acid.[2]
In 1933, Yasuhiko Asahina and J. Asano studied salazinic acid they had isolated from Parmelia cetrata, and found a unique ring system with seven members containing two phenolic components. The fundamental structure was named depsidone, that is, a seven-membered ring with an oxygen bridge binding two aromatic rings.[3] Japanese chemists demonstrated in the late 1960s that the isolated mycobiont of the lichen Ramalina crassa could produce salazinic acid when grown in laboratory culture.[4] Subsequent studies tried to determine the influence of environmental factors on the production of salazinic acid in culture. For example, two studies in the late 1980s showed that only 4-O-demethylbarbatic acid (a precursor of salazinic acid) was produced by the isolated mycobiont of Ramalina siliquosa when grown in malt yeast extract medium supplemented with low amounts of sucrose.[5][6] When extra sucrose was added to the growth medium, the production of salazinic acid was observed; the increased osmolality enhances the reaction from 4-O-demethylbarbatic acid to salazinic acid.[7]
Properties
Salazinic acid has a molecular formula of C18H12O10, and a molecular mass of 388.3 grams/mole. In its purified form, it exists as colourless needles with a melting point range between 260–268 °C (500–514 °F) that undergo a colour change to brown at about 240 °C (464 °F). Its solubility in water is about 27 milligrams per litre.[8]
The compound has been shown in in vitro studies to have antimicrobial properties,[9][10] but it did not have any substantive antimycobacterial effects when tested against Mycobacterium aurum.[11][12] Recent (2021) research indicates that salazinic acid is a potent modulator of Nrf2, NF-κB and STAT3 signaling pathways in colorectal cancer cells.[13]
The complete 1H and 13C NMR spectral assignments for salazinic acid were reported in 2000.[14]
Occurrence
Salazinic acid is derived via the acetyl polymalonyl pathway, a metabolic pathway that uses acetyl-CoA and malonyl-CoA (derivatives of coenzyme A). The compound is common in the large lichen genus Parmotrema, and plays an important role in the chemotaxonomy and systematics of that genus. A 2020 revision included 66 salazinic acid-containing species.[15] The presence or absence of the compound is also important in the classification of genus Bulbothrix.[16][17]
In nature, salazinic acid serves as an antioxidant as well as a photoprotectant, helping the lichen to survive in conditions of both abiotic and biotic stress. A study of three foliose lichen species showed higher quantities of salazinic acid correlating with increases in altitude.[18] An earlier study demonstrated other possible effects of environmental conditions on salazinic acid content. It was shown that the salazinic acid content of Ramalina siliquosa is higher where the annual mean temperature is higher, and the content of the lichens growing on the dark-coloured rock or on the southern rock face is higher than that of the lichens growing on the light-coloured rock or on the northern rock face.[19]
Related compounds
The depsidones chalybaeizanic acid and quaesitic acid, isolated from the lichens Xanthoparmelia amphixanthoides and Hypotrachyna quaesita, respectively, are structurally similar to salazinic acid.[20] In consalazinic acid, the aldehyde group of salazinic acid is replaced with a benzyl alcohol functional group.[21]
8'-O-Methylsalazinic acid was isolated from Parmotrema dilatatum.[22] Several new synthesised derivatives of salazinic acid were reported in 2021 using bromination, nucleophilic addition, Friedel-Crafts alkylation, and esterification.[23]
Eponyms
Several authors have explicitly named salazinic acid in the specific epithets of their published lichen species, thereby acknowledging the presence of this compound as an important taxonomic characteristic. These eponyms are listed here, followed by their taxonomic authority and year of publication:
- Acanthothecis salazinica van den Boom & Sipman (2013)[24]
- Bryoria salazinica Brodo & D.Hawksw. (1977)[25]
- Graphina salazinica A.W.Archer (2001)[26]
- Karoowia salazinica Hale (1989)[27]
- Lepraria salazinica Tønsberg (2007)[28]
- Myelochroa salazinica Sheng L.Wang, J.B.Chen & Elix (2001)[29]
- Ocellularia salazinica Papong, Mangold & Lücking (2014)[30]
- Pertusaria salazinica A.W.Archer & Elix (2017)[31]
- Phaeographina salazinica A.W.Archer (2003)[32]
- Psiloparmelia salazinica Elix & T.H.Nash (1992)[33]
- Diorygma salazinicum Sutjar. & Kalb (2014)[34]
- Oropogon salazinicus Essl. (1989)[35]
References
- ↑ Zopf, Wilhelm (1897). "Zur Kenntniss der Flechtenstoffe". Justus Liebigs Annalen der Chemie (in German). 297 (2): 222–256. doi:10.1002/jlac.18972950209.
- ↑ Asahina, Yasuhiko; Shibata, Shoji (1954). Chemistry of Lichen Substances. Tokyo: Japan Society for the Promotion of Science. pp. 130–134. ISBN 978-0-598-82060-0.
- ↑ Shibata, Shoji (2000). "Yasuhiko Asahina (1880–1975) and his studies on lichenology and chemistry of lichen metabolites". The Bryologist. 103 (4): 710–719. doi:10.1639/0007-2745(2000)103[0710:yaahso]2.0.co;2. S2CID 86351217.
- ↑ Komiya, Takeya; Shibata, Shoji (1969). "Formation of lichen substances by mycobionts of lichens. Isolation of (+) usnic acid and salazinic acid from mycobionts of Ramalina spp". Chemical and Pharmaceutical Bulletin. 17 (6): 1305–1306. doi:10.1248/cpb.17.1305.
- ↑ Hamada, Nobuo; Ueno, Tamio (1987). "Depside from an isolated lichen mycobiont". Agricultural and Biological Chemistry. 51 (6): 1705–1706. doi:10.1080/00021369.1987.10868233.
- ↑ Hamada, Nobuo (1989). "The effect of various culture conditions on depside production by an isolated lichen mycobiont". The Bryologist. 92 (3): 310–313. doi:10.2307/3243399. JSTOR 3243399.
- ↑ Hamada, Nobuo; Miyagawa, Hisashi (1995). "Secondary metabolites from isolated lichen mycobionts cultured under different osmotic conditions". The Lichenologist. 27 (3): 201–205. doi:10.1016/s0024-2829(95)80018-2. S2CID 84545779.
- ↑ Iskandar, I.K.; Syers, J.K. (1971). "Solubility of lichen compounds in water: pedogenetic implications". The Lichenologist. 5 (1–2): 45–50. doi:10.1017/s0024282971000082. S2CID 86701324.
- ↑ Candan, Mehmet; Yılmaz, Meral; Tay, Turgay; Erdem, Murat; Türk, Ayşen Özdemir (2007). "Antimicrobial activity of extracts of the lichen Parmelia sulcata and its salazinic acid constituent". Zeitschrift für Naturforschung C. 62 (7–8): 619–621. doi:10.1515/znc-2007-7-827. PMID 17913083. S2CID 20709081.
- ↑ Manojlovića, Nedeljko; Ranković, Branislav; Kosanić, Marijana; Vasiljević, Perica; Stanojković, Tatjana (2012). "Chemical composition of three Parmelia lichens and antioxidant, antimicrobial and cytotoxic activities of some their major metabolites". Phytomedicine. 19 (13): 1166–1172. doi:10.1016/j.phymed.2012.07.012. PMID 22921748.
- ↑ Ingólfsdóttir, Kristı́n; Chung, Gavin A.C.; Skúlason, Vilhjálmur G.; Gissurarson, Stefán R.; Vilhelmsdóttir, Margrét (1998). "Antimycobacterial activity of lichen metabolites in vitro". European Journal of Pharmaceutical Sciences. 6 (2): 141–144. doi:10.1016/s0928-0987(97)00078-x. PMID 9795033.
- ↑ Honda, N.K.; Pavan, F.R.; Coelho, R.G.; de Andrade Leite, S.R.; Micheletti, A.C.; Lopes, T.I.B.; Misutsu, M.Y.; Beatriz, A.; Brum, R.L.; Leite, C.Q.F. (2010). "Antimycobacterial activity of lichen substances". Phytomedicine. 17 (5): 328–332. doi:10.1016/j.phymed.2009.07.018. PMID 19683421.
- ↑ Papierska, Katarzyna; Krajka-Kuźniak, Violetta; Paluszczak, Jarosław; Kleszcz, Robert; Skalski, Marcin; Studzińska-Sroka, Elżbieta; Baer-Dubowska, Wanda (2021). "Lichen-derived depsides and depsidones modulate the Nrf2, NF-κB and STAT3 signaling pathways in colorectal cancer cells". Molecules. 26 (16): 4787. doi:10.3390/molecules26164787. PMC 8400444. PMID 34443375.
- ↑ Eifler-Lima, V. L.; Sperry, A.; Sinbandhit, S.; Boustie, J.; Tomasi, S.; Schenkel, E. (2000). "NMR spectral data of salazinic acid isolated from some species of Parmotrema". Magnetic Resonance in Chemistry. 38 (6): 472–474. doi:10.1002/1097-458X(200006)38:6<472::AID-MRC658>3.0.CO;2-P. S2CID 96531766.
- 1 2 Spielmann, Adriano Afonso; Marcelli, Marcelo Pinto (2020). "Type studies on Parmotrema (Parmeliaceae, Ascomycota) with salazinic acid". Plant and Fungal Systematics. 65 (2): 403–508. doi:10.35535/pfsyst-2020-0028.
- ↑ Benatti, M.N. (2012). "A review of the genus Bulbothrix Hale: the isidiate, sorediate, and pustulate species with medullary salazinic acid". Mycosphere. 4 (1): 1–30. doi:10.5943/mycosphere/4/1/1.
- ↑ Benatti, Michel (2012). "A review of the genus Bulbothrix Hale: the species with medullary salazinic acid lacking vegetative propagules". MycoKeys. 5: 1–30. doi:10.3897/mycokeys.5.3342.
- ↑ Shukla, Vertika; Patel, D.K.; Bajpai, Rajesh; Semwal, Manoj; Upreti, D.K. (2015). "Ecological implication of variation in the secondary metabolites in Parmelioid lichens with respect to altitude". Environmental Science and Pollution Research. 23 (2): 1391–1397. doi:10.1007/s11356-015-5311-z. PMID 26370809. S2CID 207276246.
- ↑ Hamada, N. (1982). "The effect of temperature on the content of the medullary depsidone salazinic acid in Ramalina siliquosa (lichens)". Canadian Journal of Botany. 60 (4): 383–385. doi:10.1139/b82-053.
- ↑ Elix, John A.; Wardlaw, Judith H. (1999). "The structure of chalybaeizanic acid and quaesitic acid, two new lichen depsidones related to salazinic acid". Australian Journal of Chemistry. 52 (7): 713–716. doi:10.1071/CH99056.
- ↑ O'Donovan, Donal G.; Roberts, George; Keogh, Myles F. (1980). "Structure of the β-orcinol depsidones, connorstictic and consalazinic acids". Phytochemistry. 19 (11): 2497–2499. doi:10.1016/s0031-9422(00)91070-7. S2CID 85350700.
- ↑ Asshaima Paramita Devi, Thuc-Huy Duong, Solenn Ferron, Mehdi A Beniddir, Minh-Hiep Dinh, Van-Kieu Nguyen, Nguyen-Kim-Tuyen Pham, Dinh-Hung Mac, Joël Boustie, Warinthorn Chavasiri, Pierre Le Pogam (2020). "Salazinic acid-derived depsidones and diphenylethers with α-glucosidase inhibitory activity from the lichen Parmotrema dilatatum". Planta Medica. 86 (16): 1216–1224. doi:10.1055/a-1203-0623. PMID 32819010. S2CID 221221650.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Pham, Nguyen-Kim-Tuyen; Tran, Nguyen-Minh-An; Truong Nguyen, Huy; Pham, Duc-Dung; Nguyen, Thi-Quynh-Trang; Nguyen, Thi-Hong-Anh; Nguyen, Huu-Tri; Do, Thanh-Hung; Nguyen, Ngoc-Hong; Duong, Thuc-Huy (2022). "Design, modification, and bio-evaluation of salazinic acid derivatives". Arabian Journal of Chemistry. 15 (1): 103535. doi:10.1016/j.arabjc.2021.103535.
- ↑ van den Boom, Pieter P.G.; Sipman, Harrie J.M. (2013). "Sixty-two species of lirelliform Graphidaceae (Ascomycota) new to Panama, with four species newly described to science". Herzogia. 26: 9–20. doi:10.13158/heia.26.1.2013.9. S2CID 84432921.
- ↑ Brodo, Irwin M.; Hawksworth, David L. (1977). Alectoria and allied genera in North America. Opera Botanica. Vol. 42. Stockholm: Lund Botanical Society. p. 130. ISBN 978-9154602117.
- ↑ Archer, A.W. (2001). "The lichen genus Graphina (Graphidaceae) in Australia: new reports and new species". Mycotaxon. 77: 176.
- ↑ Hale, M.E. (1989). "A monograph of the lichen genus Karoowia Hale". Mycotaxon. 35 (1): 189.
- ↑ Tønsberg, T. (2007). "Notes on the lichen genus Lepraria in Great Smoky Mountains National Park; southeastern North America: Lepraria lanata and L. salazinica spp. nov". Opuscula Philolichenum. 4: 51–54.
- ↑ Wang, S.L.; Chen, J.B.; Elix, J.A. (2001). "Two new species of the lichen genus Myelochroa (Parmeliaceae, Ascomycota) from China". Mycotaxon. 77: 25–30.
- ↑ Papong, Khwanruan Butsatorn; Mangold, Armin; Lücking, Robert; Lumbsch, H. Thorsten (2014). "New species and new records of thelotremoid Graphidaceae (Ascomycota: Ostropales) from Thailand". Phytotaxa. 189 (1): 238. doi:10.11646/phytotaxa.189.1.16.
- ↑ Archer, A.W.; Elix, J.A. (2017). "Seven new species and a new record in the lichen genus Pertusaria (Pertusariales, lichenized Ascomycota) from eastern Australia" (PDF). Australasian Lichenology. 80: 6.
- ↑ Archer, A.W. (2003). "New species in the lichen family Graphidaceae (Ascomycota) from Australia and the Solomon Islands". Mycotaxon. 88: 143–147.
- ↑ Elix, John A.; Nash, Thomas H. (1992). "A synopsis of the lichen genus Psiloparmelia (Ascomycotina, Parmeliaceae)". The Bryologist. 95 (4): 388. doi:10.2307/3243562. JSTOR 3243562.
- ↑ Sutjaritturakan, Jutarat; Saipunkaew, Wanaruk; Boonpragob, Kansri; Kalb, Kalb (2014). "New species of Graphidaceae (Ostropales) Lecanoromycetes) from southern Thailand". Phytotaxa. 189 (1): 312–324. doi:10.11646/PHYTOTAXA.189.1.22.
- ↑ Esslinger, Theodore L. (1989). Systematics of Oropogon (Alectoriaceae) in the New World. Systematic Botany Monographs. Vol. 28. American Society of Plant Taxonomists. p. 109. ISBN 978-0-912861-28-9.

.jpg.webp)