The salinosporamides are a group of closely related chemical compounds isolated from marine bacteria in the genus Salinispora.[1][2][3][4] They possess a densely functionalized γ-lactam-β-lactone bicyclic core.
Salinosporamide A has attracted interest for its potential use in treating various types of cancer.[5][6][7][8]
In addition, a variety of synthetic analogs have been prepared.[9]
Chemical structures
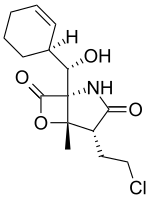
 Salinosporamide B
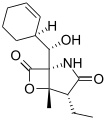
Salinosporamide B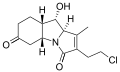 Salinosporamide C
Salinosporamide C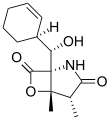 Salinosporamide D
Salinosporamide D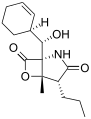 Salinosporamide E
Salinosporamide E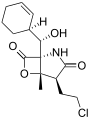 Salinosporamide F
Salinosporamide F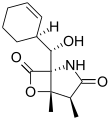 Salinosporamide G
Salinosporamide G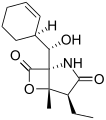 Salinosporamide H
Salinosporamide H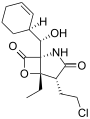 Salinosporamide I
Salinosporamide I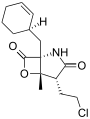 Salinosporamide J
Salinosporamide J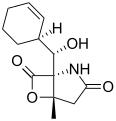 Salinosporamide K
Salinosporamide K
References
- ↑ Feling, Robert H.; Buchanan, Greg O.; Mincer, Tracy J.; Kauffman, Christopher A.; Jensen, Paul R.; Fenical, William (2003). "Salinosporamide A: A Highly Cytotoxic Proteasome Inhibitor from a Novel Microbial Source, a Marine Bacterium of the New Genus Salinospora". Angewandte Chemie International Edition. 42 (3): 355–7. doi:10.1002/anie.200390115. PMID 12548698.
- ↑ Philip G. Williams; Greg O. Buchanan; Robert H. Feling; Christopher A. Kauffman; Paul R. Jensen & William Fenical (2005). "New Cytotoxic Salinosporamides from the Marine Actinomycete Salinispora tropica". J. Org. Chem. 70 (16): 6196–6203. doi:10.1021/jo050511+. PMID 16050677.
- ↑ Reed, Katherine A.; Manam, Rama Rao; Mitchell, Scott S.; Xu, Jianlin; Teisan, Sy; Chao, Ta-Hsiang; Deyanat-Yazdi, Gordafaried; Neuteboom, Saskia T. C.; et al. (2007). "Salinosporamides D−J from the Marine ActinomyceteSalinispora tropica, Bromosalinosporamide, and Thioester Derivatives Are Potent Inhibitors of the 20S Proteasome". Journal of Natural Products. 70 (2): 269–76. doi:10.1021/np0603471. PMID 17243724.
- ↑ Eustáquio, Alessandra S.; Nam, Sang-Jip; Penn, Kevin; Lechner, Anna; Wilson, Micheal C.; Fenical, William; Jensen, Paul R.; Moore, Bradley S. (2011). "The Discovery of Salinosporamide K from the Marine Bacterium "Salinispora pacifica" by Genome Mining Gives Insight into Pathway Evolution". ChemBioChem. 12 (1): 61–4. doi:10.1002/cbic.201000564. PMC 3088357. PMID 21154492.
- ↑ Fenical, William; Jensen, Paul R.; Palladino, Michael A.; Lam, Kin S.; Lloyd, G. Kenneth; Potts, Barbara C. (2009). "Discovery and development of the anticancer agent salinosporamide A (NPI-0052)". Bioorganic & Medicinal Chemistry. 17 (6): 2175–80. doi:10.1016/j.bmc.2008.10.075. PMC 2814440. PMID 19022674.
- ↑ Lam, Kin S.; Lloyd, G. Kenneth; Neuteboom, Saskia T. C.; Palladino, Michael A.; Sethna, Kobi M.; Spear, Matthew A.; Potts, Barbara C. (2009). "Chapter 12. From Natural Product to Clinical Trials: NPI-0052 (Salinosporamide A), a Marine Actinomycete-Derived Anticancer Agent". Natural Product Chemistry for Drug Discovery. p. 355. doi:10.1039/9781847559890-00355. ISBN 978-0-85404-193-0.
- ↑ Gulder, Tobias A. M.; Moore, Bradley S. (2010). "Salinosporamide Natural Products: Potent 20 S Proteasome Inhibitors as Promising Cancer Chemotherapeutics". Angewandte Chemie International Edition. 49 (49): 9346–67. doi:10.1002/anie.201000728. PMC 3103133. PMID 20927786.
- ↑ WO 2006118973, Palladino, Michael; Potts, Barbara Christine & Macherla, Venkata Rami Reddy et al., "Methods of using heterobyclic compounds for treatment of rectal cancer", published 2006-11-09, assigned to Nereus Pharmaceuticals Inc.
- ↑ Nett, Markus; Gulder, Tobias A. M.; Kale, Andrew J.; Hughes, Chambers C.; Moore, Bradley S. (2009). "Function-Oriented Biosynthesis of β-Lactone Proteasome Inhibitors inSalinispora tropica". Journal of Medicinal Chemistry. 52 (19): 6163–7. doi:10.1021/jm901098m. PMC 2771571. PMID 19746976.
External links
 Media related to Salinosporamides at Wikimedia Commons
Media related to Salinosporamides at Wikimedia Commons
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.