 | |
| Names | |
|---|---|
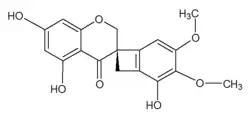
| Preferred IUPAC name
(3R)-1′,5,7-Trihydroxy-2′,3′-dimethoxy-2H,4H-spiro[[1]benzopyran-3,6′-bicyclo[4.2.0]octane]-1′(8′),2′,4′-trien-4-one | |
| Other names
(+)-Scillavone A | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C18H16O7 | |
| Molar mass | 344.31 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Scillavone A is a homoisoflavone that can be isolated from the bulbs of Scilla scilloides[1] (Barnardia japonica).
References
- ↑ Nishida, Yoichiro; Eto, Masashi; Miyashita, Hiroyuki; Ikeda, Tsuyoshi; Yamaguchi, Koki; Yoshimitsu, Hitoshi; Nohara, Toshihiro; Ono, Masateru (2008). "A new homostilbene and two new homoisoflavones from the bulbs of Scilla scilloides". Chemical and Pharmaceutical Bulletin. 56 (7): 1022–1025. doi:10.1248/cpb.56.1022. PMID 18591825.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.