| |||
| |||
| Names | |||
|---|---|---|---|
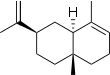
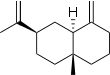
| IUPAC names
α: Eudesma-3,11-diene β: Eudesma-4(14),11-diene γ: Eudesma-4(14),7(11)-diene δ: Eudesma-4,6-diene | |||
| Systematic IUPAC name
α: (2R,4aR,8aR)-2-Isopropenyl-4a,8-dimethyl-1,2,3,4,4a,5,6,8a-octahydronaphthalene β: (4aR,7R,8aS)-7-Isopropenyl-4a-methyl-1-methylenedecahydronaphthalene γ: (4aR,8aS)-7-Isopropylidene-4a-methyl-1-methylenedecahydronaphthalene δ: (3R,4aS,5R,8aS)-3-Isopropyl-5,8a-dimethyl-1,2,3,4,4a,5,6,8a-octahydronaphthalene | |||
| Other names
Selina-3,11-diene (α); beta-Eudesmene (β) | |||
| Identifiers | |||
| |||
3D model (JSmol) |
| ||
| ChemSpider | |||
PubChem CID |
|||
| UNII |
| ||
| |||
| |||
| Properties | |||
| C15H24 | |||
| Molar mass | 204.357 g·mol−1 | ||
| Density | α: 0.914 g/cm3 (20 °C)[1] β: 0.915 g/cm3 (20 °C)[2] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Selinenes are a group of closely related isomeric chemical compounds which are classified as sesquiterpenes. The selinenes all have the molecular formula C15H24 and they have been isolated from a variety of plant sources. α-Selinene and β-selinene are the most common and are two of the principal components of the oil from celery seeds.[3] γ-Selinene and δ-selinene are less common.
References
- ↑ Ruzicka, L.; Stoll, M. (1923). "Höhere Terpenverbindungen XIV. Zur Kenntnis des Selinens und der Sesquiterpenalkohole des Selleriesamenöls". Helvetica Chimica Acta. 6: 846–855. doi:10.1002/hlca.19230060192.
- ↑ Tyagi, B. S.; Ghatge, B.B.; Bhattacharyya, S.C. (1963). "Terpenoids—XLIII". Tetrahedron. 19 (7): 1189–1193. doi:10.1016/S0040-4020(01)98579-5.
- ↑ Mathew Attokaran (Jan 13, 2011). Natural Food Flavors and Colorants. John Wiley & Sons. ISBN 9780470959114.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.

-Delta-selinene.svg.png.webp)