 | |
| Clinical data | |
|---|---|
| Trade names | Senvelgo |
| License data | |
| Routes of administration | By mouth |
| ATCvet code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
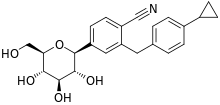
| Formula | C23H25NO5 |
| Molar mass | 395.455 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Velagliflozin, sold under the brand name Senvelgo, is an antidiabetic medication used for the treatment of cats.[1][2] Velagliflozin is a sodium-glucose cotransporter 2 (SGLT2) inhibitor.[3] It is taken by mouth.[1]
Medical uses
Velagliflozin is indicated to improve glycemic control in otherwise healthy cats with diabetes not previously treated with insulin.[1][2][3]
References
- 1 2 3 4 "Senvelgo- velagliflozin solution". DailyMed. 8 November 2023. Retrieved 13 December 2023.
- 1 2 https://animaldrugsatfda.fda.gov/adafda/app/search/public/document/downloadFoi/14320
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - 1 2 "Dear Veterinarian Letter regarding important safety conditions associated with the use of Senvelgo (velagliflozin oral solution) for improving glycemic control in certain cats with diabetes mellitus". U.S. Food and Drug Administration. 4 December 2023. Retrieved 13 December 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.