Separation of isotopes by laser excitation (SILEX) is a process under development to enrich uranium on an industrial scale for nuclear reactors. It is strongly suspected that it utilizes laser condensation repression to excite the uranium-235 isotope in uranium hexafluoride (UF6), allowing this lighter molecule to move more rapidly to the outer rim of a gaseous jet and resist condensing compared to the heavier, unexcited 238UF6.[1] This differs greatly from previous methods of laser enrichment explored for their commercial prospects: one using atomic uranium (Atomic Vapor Laser Isotope Separation (AVLIS)) and another molecular method that uses lasers to dissociate a fluorine atom from 235UF6 (Molecular Laser Isotope Separation (MLIS)), allowing the enriched product to precipitate out as a solid.[1]
While the Australian company Silex Systems Limited is the most prominent developer of this technology (as part of the Global Laser Enrichment consortium), the acronym SILEX really only refers to a physical separation concept utilizing condensation repression that is well known and under development or being used for multiple applications around the world.[2] Slight variations in operating parameters, equipment arrangements, lasers and their capabilities, may exist from one SILEX-type process to the next (and be called by different names), but the physical separation concept remains the same if condensation repression is utilized, especially when compared to that used by AVLIS or MLIS.
Princeton physicist Ryan Snyder has suggested that this process may lead to the further proliferation of nuclear weapons due to its increasingly accessible technological pathway[2][3] and undetectable signatures (small area footprint and high energy efficiency).[1]
History
Development of various MLIS variants began already in the 1970s. In most of them, an infrared laser vibrationally excited one of the isotopes such as 235UF6 of gaseous uranium hexafluoride. This requires a wavelength near 16 µm. The excited molecules were then further excited up to dissociation, either again at 16 µm or by a UV laser.
After initial euphoria, LIS of uranium was mostly abandoned during the 1990s. Urenco published in 1992 the reasons for their decision.[4] One reason was that LIS processes seemed to require a number of further developments of uncertain outcome, whereas centrifuges had reached technical maturity around this time. But in Australia the SILEX variant was not discontinued.
In November 1996, Silex Systems Limited licensed its technology exclusively to United States Enrichment Corporation (USEC) for uranium enrichment.[5]
In 1999, the United States signed the Agreement for Cooperation between the Government of Australia and the Government of the United States of America concerning Technology for the Separation of Isotopes of Uranium by Laser Excitation [SILEX Agreement], which allowed cooperative research and development between the two countries on the SILEX process.[6]
Silex Systems concluded the second stage of testing in 2005 and began its Test Loop Program. Already before, in 2003, USEC backed out from the project. In 2007, Silex Systems signed an exclusive commercialization and licensing agreement with General Electric Corporation (GE). The Test Loop Program was transferred to GE's facility in Wilmington, North Carolina. Also in 2007, GE Hitachi Nuclear Energy (GEH) signed letters of intent for uranium enrichment services with Exelon and Entergy - the two largest nuclear power utilities in the USA.[7]
In 2008, GEH spun off Global Laser Enrichment (GLE) to commercialise the SILEX Technology and announced the first potential commercial uranium enrichment facility using the Silex process. The U.S. Nuclear Regulatory Commission (NRC) approved a license amendment allowing GLE to operate the Test Loop. Also in 2008, Cameco Corporation, Canada, the world's largest uranium producer, joined GE and Hitachi as a part owner of GLE.[8]
In 2010, concerns were raised that the SILEX process poses a threat to global nuclear security (see Proliferation concerns).[9]
In August 2011, GLE applied to the NRC for a permit to build a commercial plant at Wilmington, which would enrich uranium to a maximum of 8% 235U.[10] On September 19, 2012, the NRC made its initial decision on GLE's application, and granted the requested permit.[11] Silex completed its phase 1 test loop program at GE-Hitachi Global Laser Enrichment's (GLE) facility in Wilmington. The commercial plant's target enrichment level is 8 percent, which puts it on the upper end of low-enriched uranium.[12]
In 2014, both GLE and Silex Systems restructured, with Silex halving its workforce.[13] In 2016 GEH withdrew from GLE, writing-off their investment.[13][14]
In 2016, the United States Department of Energy agreed to sell about 300,000 tonnes of depleted uranium hexafluoride to GLE for re-enrichment (from 0.35 to 0.7 % 235U) using the SILEX process over 40 years at a proposed Paducah, Kentucky Laser Enrichment Facility.[15]
In 2018, Silex Systems abandoned its plans for GLE, intending to repatriate the SILEX technology to Australia.[16]
In 2021, Silex Systems took majority ownership (51%) of GLE, with Cameco (49%) as minority owner. The path to market for the venture is underpinned by an agreement between GLE and the US Department of Energy under which DOE uranium tailings will be made available for the proposed Paducah Laser Enrichment project. Silex's technology will be used to produce natural grade uranium from the tailings.[17] GLE intends now (from 2022) to focus on re-enrichment in Paducah.[18] It is on the site, where the last diffusion plant for uranium enrichment worked until 2013. It left behind several hundred thousand tons of depleted UF6.
Process
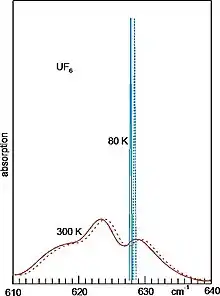
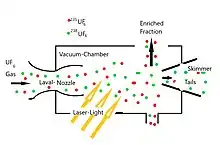
The shortest-wavelength fundamental vibration of gaseous UF6 is around 16 µm. At room temperature its width (around 20 cm−1) is much larger than the isotopic shift (0.6 cm−1). The broadening is due to thermally populated excited vibrational and rotational states. To allow for selective excitation, the UF6, diluted about 100 fold by a carrier gas (which can be argon or nitrogen), is cooled to about 80 K by adiabatic expansion through a nozzle into vacuum. Initially there are still collisions (which are necessary for cooling). But after traveling about 10 nozzle diameters, due to the expansion, they are so rare that condensation can no longer take place. Avoiding collisions is also necessary to suppress any collisional transfer of energy between the isotopes. Such a molecular beam method is used in all cases, where spectral narrowing is needed for selective excitation.
With SILEX, the pressure and nozzle diameter are chosen large enough to provide a sufficient number of collisions immediately after the nozzle, to allow for formation of clusters (UF6•G) with the carrier gas G. (UF6•UF6 clusters are practically not formed due to the much lower density of UF6 compared to G.) If 235UF6 is selectively excited at 628.3 cm−1, then this molecule does not aggregate with G, whereas the nonexcited heavier 238UF6 does. Due to their higher thermal velocity, the free molecules leave the axis of the molecular beam faster than the clusters. The latter are therefore enriched in the part transmitted by a skimmer nozzle downstream, whereas the non-transmitted fraction is enriched in the 235UF6. The enrichment factor is the better, the larger the transmitted fraction (i.e. the smaller the depletion and the smaller the cut). That is, SILEX uses a separation nozzle, modified by a laser and profiting from selective repression of cluster formation ("condensation").
For that, the CO2 laser needs at least 20 MW. With a Raman shift of 354.3 cm−1 and a CO2 laser wavenumber of 982.1 cm−1 (10R30 line), one receives 627.8 cm−1. This is only close to the Q-branch of 235UF6 (center at 628.3 cm−1, width 0.01 cm−1 [19]) and is even closer to the Q-branch of 238UF6. GLE does not inform, how they do the necessary fine tuning. High-pressure CO2 lasers would cause additional problems with the pulse repetition rate. With common (atmospheric-pressure) CO2 lasers and with the stimulated Raman shifter the state of technology is 2–4 kHz.[20] In order not to leave large parts of the molecular beam unirradiated, one needs at least 20 kHz (according to Urenco several tens of kHz[21]), unless pulsed nozzles are used. The nozzles themselves must have slit form, in order to provide enough absorption length.
GLE informs that they reach separation factors of 2–20, the higher values probably coupled to a poorer depletion (which is not given). This is sufficient for enrichment from natural uranium (0,72 % 235U) to reactor grade (> 3% 235U). The pioneer works of the van den Bergh group obtained only much smaller enrichments with SF6.[22]
Using other lasers with suitable wavelengths, SILEX can also be used for the isotopic enrichment of other elements such as chlorine, molybdenum, carbon and silicon.
Proliferation concerns
Compared to current enrichment technologies, SILEX obtains a higher enrichment. Hence fewer stages are necessary to reach bomb grade uranium (> 90% 235U). According to GLE, each stage requires as little as 25% of the space of the conventional methods. Hence it would facilitate to rogue governments to hide a production facility for bomb uranium.[9] The attractiveness is even enhanced by the claims of GLE that a SILEX plant is faster and cheaper to build, and consumes considerably less energy. Scientists therefore expressed their concerns repeatedly that SILEX could create an easy path towards a nuclear weapon (see e.g.[23]). The model calculations of Ryan Snyder substantiate these warnings.[1]
Security classification
SILEX is the only privately held information that is classified by the U.S. government. In June 2001, the U.S. Department of Energy classified "certain privately generated information concerning an innovative isotope separation process for enriching uranium". Under the Atomic Energy Act, all information not specifically declassified is classified as Restricted Data, whether it is privately or publicly held. This is in marked distinction to the national security classification executive order, which states that classification can only be assigned to information "owned by, produced by or for, or is under the control of the United States Government". This is the only known case of the Atomic Energy Act being used in such a manner.[24][25]
Popular culture
The 2014 Australian Broadcasting Corporation drama The Code uses "Laser Uranium Enrichment" as a core plot device. The female protagonist Sophie Walsh states that the technology will be smaller, less energy-intensive, and more difficult to control once it is a viable alternative to current methods of enrichment. Ms. Walsh also states that the development of the technology has been protracted, and that there are significant governmental interests in maintaining the secrecy and classified status of the technology.
See also
References
- 1 2 3 4 Snyder, Ryan (2016-05-03). "A Proliferation Assessment of Third Generation Laser Uranium Enrichment Technology". Science & Global Security. 24 (2): 68–91. doi:10.1080/08929882.2016.1184528. ISSN 0892-9882.
- 1 2 Snyder, Ryan (2021-05-18). "Proliferation Risks of Laser Enrichment of Uranium". National Academy of Sciences.
- ↑ W. Eberhardt (DESY), W. Fuss (MPQ), F. Lehner (DESY), and R. Snyder (IFSH) (2019-11-04). "FELs and Laser Isotope Separation". Deutsches Elektronen-Synchrotron (DESY) and European XFEL.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ↑ K.R., Schneider (1995). "LIS: the view from Urenco".
{{cite journal}}: Cite journal requires|journal=(help) - ↑ "Silex Systems Ltd: New Laser Technology for Uranium Enrichment". Sustainable Energy & Anti-Uranium Service Inc. Archived from the original on 2007-05-14. Retrieved 2006-04-21.
- ↑ “Agreement for Cooperation between the Government of Australia and the Government of the United States of America concerning Technology for the Separation of Isotopes of Uranium by Laser Excitation (SILEX Agreement), Agreed Minute and Exchange of Notes (Washington, 28 October 1999). ATS 19 of 2000”. Australasian Legal Information Institute, Australian Treaties Library. Retrieved on 15 April 2017.
- ↑ "The Biggest Nuclear Operators In The United States". Investopedia US. 2011-03-28. Archived from the original on 2012-11-07. Retrieved 2012-08-28.
- ↑ "Cameco Joins GE Hitachi Enrichment Venture". Cameco. 2008-06-20. Archived from the original on 2012-08-09. Retrieved 2012-08-28.
- 1 2 McMurtrie, Craig (2010-04-13). "Australian laser 'threatens nuclear security'". ABC Online. Archived from the original on 2012-08-29. Retrieved 2012-08-28.
- ↑ Broad, William J. (2011-08-20). "Laser Advances in Nuclear Fuel Stir Terror Fear". The New York Times. Archived from the original on 2012-11-03. Retrieved 2012-08-28.
- ↑ Nuclear Regulatory Commission announcement |date=2012-09-19| http://pbadupws.nrc.gov/docs/ML1226/ML12263A046.pdf
- ↑ "Lasers point to the future of uranium enrichment". Gizmag.com. 6 November 2013. Retrieved 2013-11-06.
- 1 2 Patel, Sonal (1 June 2016). "GE-Hitachi Exits Nuclear Laser-Based Enrichment Venture". POWER. Retrieved 1 April 2017.
- ↑ Yasuhara, Akiko (31 March 2017). "Toshiba's U.S. unit bankruptcy dims Japan's nuclear ambitions". The Japan Times. Retrieved 1 April 2017.
- ↑ "US DOE sells depleted uranium for laser enrichment". World Nuclear News. 2016-11-11. Retrieved 2016-11-15.
- ↑ "Silex Systems out of GLE restructure". World Nuclear News. 13 June 2018. Retrieved 14 June 2018.
- ↑ Silex gets go ahead to enrich stockpiles to enrich uranium, AuManufacturing, 19 Jan 2021.
- ↑ "Global Laser Enrichment | Silex".
- ↑ Takami, Michio; Oyama, Toshiyuki; Watanabe, Tsunao; Namba, Susumu; Nakane, Ryohei (1984-02-01). "Cold Jet Infrared Absorption Spectroscopy: The ν 3 Band of UF 6". Japanese Journal of Applied Physics. 23 (2A): L88. doi:10.1143/JJAP.23.L88. ISSN 0021-4922. S2CID 93245695.
- ↑ Ronander, Einar; Rohwer, Erich G. (1993-05-04). Fotakis, Costas; Kalpouzos, Costas; Papazoglou, Theodore G. (eds.). "Multikilowatt TEA-CO2 laser system for molecular laser isotope separation". 9th International Symposium on Gas Flow and Chemical Lasers. 1810. Heraklion, Greece: 49–52. doi:10.1117/12.144664. S2CID 94250559.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ k. r., Schneider (1995). "LIS: The view from Urenco".
- ↑ Zellweger, J. -M.; Philippoz, J. -M.; Melinon, P.; Monot, R.; van den Bergh, H. (1984-03-19). "Isotopically Selective Condensation and Infrared-Laser-Assisted Gas-Dynamic Isotope Separation". Physical Review Letters. 52 (12): 1055. doi:10.1103/PhysRevLett.52.1055. ISSN 0031-9007.
- ↑ Boureston, Jack; Ferguson, Charles D. (2005-03-01). "Laser Enrichment: Separation anxiety". Bulletin of the Atomic Scientists. 61 (2): 14–18. doi:10.2968/061002005. ISSN 0096-3402.
- ↑ Steven Aftergood (26 June 2001). "DOE classifies privately held info". Secrecy News, Federation of American Scientists. Retrieved 2007-08-23.
- ↑ Steven Aftergood (23 August 2007). "A glimpse of the SILEX uranium enrichment process". Secrecy News, Federation of American Scientists. Retrieved 2007-08-23.
External links
- Silex Systems Limited: http://www.silex.com.au/
- Snyder, R., "A Proliferation Assessment of Third Generation Laser Uranium Enrichment Technology", Science & Global Security: https://doi.org/10.1080/08929882.2016.1184528