 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C34H38O13 |
| Molar mass | 654.7 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
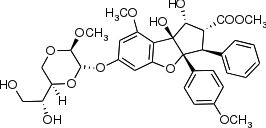
Silvestrol is a natural product from the flavagline family, with a cyclopenta[b] benzofuran core structure and an unusual dioxane ether side chain, which is found in the bark of trees from the genus Aglaia, especially Aglaia silvestris and Aglaia foveolata.[1]
Bioactivity
It acts as a potent and selective inhibitor of the RNA helicase enzyme eIF4A, and has both broad-spectrum antiviral activity against diseases such as Ebola and coronaviruses, [2][3][4][5][6] and anti-cancer properties,[7][8] which makes it of considerable interest in medical research. However, as it cannot be extracted from tree bark in commercial amounts and is prohibitively complex to produce synthetically,[9] practical applications have focused more on structurally simplified analogues such as CR-31-B.[10]
See also
References
- ↑ Pan L, Woodard JL, Lucas DM, Fuchs JR, Kinghorn AD (July 2014). "Rocaglamide, silvestrol and structurally related bioactive compounds from Aglaia species". Natural Product Reports. 31 (7): 924–39. doi:10.1039/c4np00006d. PMC 4091845. PMID 24788392.
- ↑ Biedenkopf N, Lange-Grünweller K, Schulte FW, Weißer A, Müller C, Becker D, et al. (January 2017). "The natural compound silvestrol is a potent inhibitor of Ebola virus replication". Antiviral Research. 137: 76–81. doi:10.1016/j.antiviral.2016.11.011. PMID 27864075. S2CID 205577158.
- ↑ Elgner F, Sabino C, Basic M, Ploen D, Grünweller A, Hildt E (March 2018). "Inhibition of Zika Virus Replication by Silvestrol". Viruses. 10 (4): 149. doi:10.3390/v10040149. PMC 5923443. PMID 29584632.
- ↑ Müller C, Schulte FW, Lange-Grünweller K, Obermann W, Madhugiri R, Pleschka S, et al. (February 2018). "Broad-spectrum antiviral activity of the eIF4A inhibitor silvestrol against corona- and picornaviruses". Antiviral Research. 150: 123–129. doi:10.1016/j.antiviral.2017.12.010. PMC 7113723. PMID 29258862.
- ↑ Henss L, Scholz T, Grünweller A, Schnierle BS (October 2018). "Silvestrol Inhibits Chikungunya Virus Replication". Viruses. 10 (11): 592. doi:10.3390/v10110592. PMC 6266838. PMID 30380742.
- ↑ Pillaiyar T, Meenakshisundaram S, Manickam M (April 2020). "Recent discovery and development of inhibitors targeting coronaviruses". Drug Discovery Today. 25 (4): 668–688. doi:10.1016/j.drudis.2020.01.015. PMC 7102522. PMID 32006468.
- ↑ Kogure T, Kinghorn AD, Yan I, Bolon B, Lucas DM, Grever MR, Patel T (2013). "Therapeutic potential of the translation inhibitor silvestrol in hepatocellular cancer". PLOS ONE. 8 (9): e76136. Bibcode:2013PLoSO...876136K. doi:10.1371/journal.pone.0076136. PMC 3784426. PMID 24086701.
- ↑ Pelletier J, Graff J, Ruggero D, Sonenberg N (January 2015). "Targeting the eIF4F translation initiation complex: a critical nexus for cancer development". Cancer Research. 75 (2): 250–63. doi:10.1158/0008-5472.CAN-14-2789. PMC 4299928. PMID 25593033.
- ↑ El Sous M, Khoo ML, Holloway G, Owen D, Scammells PJ, Rizzacasa MA (2007). "Total synthesis of (-)-episilvestrol and (-)-silvestrol". Angewandte Chemie. 46 (41): 7835–8. doi:10.1002/anie.200702700. PMID 17823902.
- ↑ Müller C, Obermann W, Schulte FW, Lange-Grünweller K, Oestereich L, Elgner F, et al. (January 2020). "Comparison of broad-spectrum antiviral activities of the synthetic rocaglate CR-31-B (-) and the eIF4A-inhibitor Silvestrol". Antiviral Research. 175: 104706. doi:10.1016/j.antiviral.2020.104706. PMC 7114339. PMID 31931103.