 | |
| Names | |
|---|---|
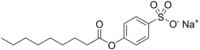
| Preferred IUPAC name
Sodium 4-(nonanoyloxy)benzene-1-sulfonate | |
| Other names
4-Sulfophenyl nonanoate sodium salt; Sodium p-nonanoyloxybenzenesulfonate; p-(Nonanoyloxy)benzenesulfonic acid sodium salt; p-Sodiosulfophenyl nonanoate | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | NOBS |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H21NaO5S | |
| Molar mass | 336.38 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Sodium nonanoyloxybenzenesulfonate (NOBS) is an important component of laundry detergents and bleaches. It is known as a bleach activator for active oxygen sources, allowing formulas containing hydrogen peroxide releasing chemicals (specifically sodium perborate, sodium percarbonate, sodium perphosphate, sodium persulfate, and urea peroxide) to effect bleaching at lower temperatures.[1]
Synthesis
NOBS is formed by the reaction of nonanoic acid (or its esters) with phenol followed by aromatic sulfonation using SO3 to form a sulfonic acid at the para-position.
Bleach activation
NOBS was developed by Procter & Gamble in 1983[2] and was first used in American laundry detergents in 1988.[3] NOBS is the main bleach activator used in the U.S.A. and Japan.[4] Compared to TAED, which is the predominant bleach activator used in Europe, NOBS is efficient at much lower temperatures. At 20 °C NOBS is 100 times more soluble than TAED in water.[5] When attacked by the perhydroxyl anion (from hydrogen peroxide), NOBS forms the peroxy acid peroxynonanoic acid and releases the leaving group sodium 4-hydroxybenzene sulfonate, which is an inert by-product.
References
- ↑ Kuzel, P.; Lieser, T. (1990). "Bleach systems". Tenside, Surfactants, Detergents. 27 (1): 23–8. doi:10.1515/tsd-1990-270109. S2CID 235325050.
- ↑ Chung, S. Y.; Spadini, G. L. (1983). US4412934.
- ↑ Arno Cahn (30 January 1994). Proceedings of the 3rd World Conference on Detergents: Global Perspectives. The American Oil Chemists Society. pp. 64–70. ISBN 978-0-935315-52-3.
- ↑ Hirschen, M. (2005). Handbook of Detergents Part C: Analysis. Marcel Dekker. pp. 439–470. ISBN 9780824703516.
- ↑ Reinhardt, G.; Borchers, G. (2009). Handbook of Detergents, Part E: Applications. CRC Press. ISBN 9781574447576.