 | |
| Names | |
|---|---|
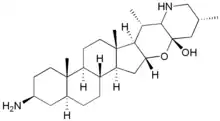
| IUPAC name
3β-Amino-16,23-epoxy-16α,28-seco-5α-solanidan-23β-ol | |
| Systematic IUPAC name
(2S,4aS,3bS,6aS,6bR,7S,7aR,10R,11aS,12aR,13aS,13bR,15aS)-2-Amino-4a,6a,7,10-tetramethyldocosahydronaphtho[2′′,1′′:4′,5′]indeno[1′,2′:5,6]pyrano[3,2-b]pyridin-11a(1H)-ol | |
| Other names
3β-Amino-22,26-epimino-16α,23-epoxy-5α,22αH,25βH-cholestan-23β-ol; (3β,5α,16α,22α,23β,25β)-3-Amino-16,23-epoxy-16,28-secosolanidan-23-ol | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C27H46N2O2 | |
| Molar mass | 430.666 |
| Appearance | long flat colorless prisms (ethanol-H2O)[1] |
| Melting point | 222 °C (432 °F; 495 K)[1] 216-217 °C [2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Solanocapsine is a toxic steroidal alkaloid from Solanum pseudocapsicum (Jerusalem cherry).
References
- 1 2 Barger, L. G.; Fraenkel-Conrat, H. L. (1936). "337. Alkaloids from Solanum pseudocapsicum, L.". Journal of the Chemical Society. 1936: 1537–1542. doi:10.1039/JR9360001537.
- ↑ Schlittler, E.; Uehlinger, H. (1952). "Über das Sterinalkaloid Solanocapsin". Helvetica Chimica Acta (in German). 35 (6): 2034–2044. doi:10.1002/hlca.19520350633.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.