 | |
| Names | |
|---|---|
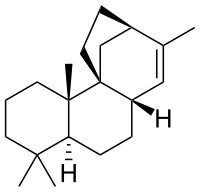
| IUPAC name
13-Methyl-9,12β-ethano-9β-podocarp-13-ene | |
| Systematic IUPAC name
(4aS,6aS,9R,11aR,11bS)-4,4,8,11b-Tetramethyl-1,2,3,4,4a,5,6,6a,9,10,11,11b-dodecahydro-9,11a-methanocyclohepta[a]naphthalene | |
| Other names
(+)-Stemar-13-ene | |
| Identifiers | |
3D model (JSmol) |
|
| 5334696 | |
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| |
| |
| Properties | |
| C20H32 | |
| Molar mass | 272.476 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Stemarene is a diterpene hydrocarbon can be produced biosynthetically through enzyme extracts from rice.[1]
References
- ↑ Mohan, Ram S.; Yee, Nathan K.N.; Coates, Robert M.; Ren, Yue-Ying; Stamenkovic, Predrag; Mendez, Irena; West, Charles A. (1996). "Biosynthesis of Cyclic Diterpene Hydrocarbons in Rice Cell Suspensions: Conversion of 9,10-syn-Labda-8(17),13-dienyl Diphosphate to 9β-Pimara-7,15-diene and Stemar-13-ene". Archives of Biochemistry and Biophysics. 330 (1): 33–47. doi:10.1006/abbi.1996.0223. PMID 8651702.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.