 | |
| Names | |
|---|---|
| IUPAC name
1,3,4,6-Tetra-O-propionyl-β-D-fructofuranosyl 2,3,4,6-tetra-O-propionyl-α-D-glucopyranoside | |
| Other names
sucrose octapropanoate, octapropionyl sucrose | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C36H54O19 | |
| Appearance | colorless crystalline solid |
| Density | 1.185 g/L [1] |
| Melting point | 45.4 °C (113.7 °F; 318.5 K)[1] |
| Boiling point | 280–290 °C (536–554 °F; 553–563 K) at 0.05 torr [2] |
| less than 0.1 g/L | |
| Solubility | ethanol, isopropanol, toluene, acetone[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
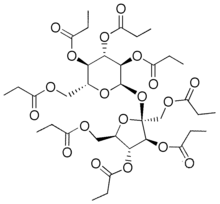
Sucrose octapropionate is a chemical compound with formula C
36H
54O
19 or (C
3H
5O
2)
8(C
12H
14O
3), an eight-fold ester of sucrose and propionic acid. Its molecule can be described as that of sucrose C
12H
22O
11 with its eight hydroxyl groups HO– replaced by propionate groups H
3C–CH
2–CO
2–. It is a crystalline colorless solid.[1] It is also called sucrose octapropanoate or octapropionyl sucrose.
History
The preparation of sucrose octapropionate was first described in 1933 by Gerald J. Cox and others.[1]
Preparation
The compound can be prepared by the reaction of sucrose with propionic anhydride in the melt state[1] or at room temperature, over several days, in anhydrous pyridine.[3]
Properties
Sucrose octapropionate is only slightly soluble in water (less than 0.1 g/L) but is soluble in many common organic solvents such as isopropanol and ethanol, from which it can be crystallized by evaporation of the solvent.[3][4]
The crystalline form melts at 45.4–45.5 °C into a viscous liquid (47.8 poises at 48.9 °C), that becomes a clear glassy solid on cooling, but easily recrystallizes.[1][3]
The density of the glassy form is 1.185 kg/L (at 20 °C). It is an optically active compound with [α]20D +53°.[3]
The compound can be vacuum distilled at 280–290 °C and 0.05 to 0.07 torr.[2]
Applications
Distillation of fully esterified propionates has been proposed as a method for the separation and identification of sugars.[2]
While the crystallinity of the pure compound prevents its use as a plasticizer it was found that incompletely esterified variants (with 1 to 2 remaining hydroxyls per molecule) will not crystallize, and therefore can be considered for that application.[5]
See also
References
- 1 2 3 4 5 6 7 Cox, Gerald J.; Ferguson, John H.; Dodds, Mary L. (1933). "III. Technology of Sucrose Octaauml;cetate and Homologous Esters". Industrial & Engineering Chemistry. 25 (9): 968–970. doi:10.1021/ie50285a006.
- 1 2 3 Hurd, Charles D.; Liggett, R. W. (1941). "Analytical Separation of Sugars by Distillation of their Propionates". Journal of the American Chemical Society. 63 (10): 2659–2662. doi:10.1021/ja01855a041.
- 1 2 3 4 Hurd, Charles D.; Gordon, K. M. (1941). "Propionyl Derivatives of Sugars". Journal of the American Chemical Society. 63 (10): 2657–2659. doi:10.1021/ja01855a040.
- ↑ Hurd, Charles D.; Liggett, R. W.; Gordon, K. M. (1941). "Distillation of Sugar Propionates at Low Pressures". Journal of the American Chemical Society. 63 (10): 2656–2657. doi:10.1021/ja01855a039.
- ↑ George P Touey and Herman E Davis (1962) "Non-crystallizing sucrose lower fatty acid esters and compositions thereof" U.S. Patent 3,057,743.