| General anaesthesia | |
|---|---|
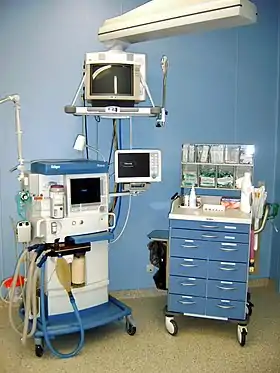 Equipment used for anaesthesia in the operating theatre | |
| Specialty | Anaesthetics |
| Uses | Facilitating surgery, terminal sedation[1] |
| Complications | Anaesthesia awareness,[2] overdose,[3] death[4] |
| MeSH | D000768 |
| MedlinePlus | 007410 |
General anaesthesia (UK) or general anesthesia (US) is a method of medically inducing loss of consciousness that renders a patient unarousable even with painful stimuli.[5] This effect is achieved by administering either intravenous or inhalational general anaesthetic medications, which often act in combination with an analgesic and neuromuscular blocking agent. Spontaneous ventilation is often inadequate during the procedure and intervention is often necessary to protect the airway.[5] General anaesthesia is generally performed in an operating theater to allow surgical procedures that would otherwise be intolerably painful for a patient, or in an intensive care unit or emergency department to facilitate endotracheal intubation and mechanical ventilation in critically ill patients. Regardless of whether a patient may prefer to be unconscious or not, certain pain stimuli could result in involuntary responses from the patient (such as movement or muscle contractions) that may make an operation extremely difficult. Thus, for many procedures, general anaesthesia is required from a practical perspective.
A variety of drugs may be administered, with the overall goal of achieving unconsciousness, amnesia, analgesia, loss of reflexes of the autonomic nervous system, and in some cases paralysis of skeletal muscles. The optimal combination of anesthetics for any given patient and procedure is typically selected by an anaesthetist, or another provider such as a nurse anaesthetist (depending on local practice and law), in consultation with the patient and the surgeon, dentist, or other practitioner performing the operative procedure.[6]
History
Attempts at producing a state of general anaesthesia can be traced throughout recorded history in the writings of the ancient Sumerians, Babylonians, Assyrians, Egyptians, Greeks, Romans, Indians, and Chinese. During the Middle Ages, scientists and other scholars made significant advances in the Eastern world, while their European counterparts also made important advances.
The Renaissance saw significant advances in anatomy and surgical technique. However, despite all this progress, surgery remained a treatment of last resort. Largely because of the associated pain, many patients chose certain death rather than undergo surgery. Although there has been a great deal of debate as to who deserves the most credit for the discovery of general anaesthesia, several scientific discoveries in the late 18th and early 19th centuries were critical to the eventual introduction and development of modern anaesthetic techniques.[7]
Two enormous leaps occurred in the late 19th century, which together allowed the transition to modern surgery. An appreciation of the germ theory of disease led rapidly to the development and application of antiseptic techniques in surgery. Antisepsis, which soon gave way to asepsis, reduced the overall morbidity and mortality of surgery to a far more acceptable rate than in previous eras.[8] Concurrent with these developments were the significant advances in pharmacology and physiology which led to the development of general anaesthesia and the control of pain. On 14 November 1804, Hanaoka Seishū, a Japanese surgeon, became the first person on record to successfully perform surgery using general anaesthesia.[9]
In the 20th century, the safety and efficacy of general anaesthesia was improved by the routine use of tracheal intubation and other advanced airway management techniques. Significant advances in monitoring and new anaesthetic agents with improved pharmacokinetic and pharmacodynamic characteristics also contributed to this trend. Finally, standardized training programs for anaesthesiologists and nurse anaesthetists emerged during this period.
Purpose
General anaesthesia has many purposes and is routinely used in almost all surgical procedures. An appropriate surgical anesthesia should include the following goals:
- Hypnosis/Unconsciousness (loss of awareness)
- Analgesia (loss of response to pain)
- Amnesia (loss of memory)
- Immobility (loss of motor reflexes)
- Paralysis (skeletal muscle relaxation and normal muscle relaxation)[3]
Instead of receiving continuous deep sedation, such as via benzodiazepines, dying patients may choose to be completely unconscious as they die.[1]
Biochemical mechanism of action
The biochemical mechanism of action of general anaesthetics is still controversial.[10] Theories need to explain the function of anaesthesia in animals and plants.[11] To induce unconsciousness, anaesthetics have myriad sites of action and affect the central nervous system (CNS) at multiple levels. General anaesthesia commonly interrupts or changes the functions of CNS components including the cerebral cortex, thalamus, reticular activating system, and spinal cord. Current theories on the anaesthetized state identify not only target sites in the CNS but also neural networks and arousal circuits linked with unconsciousness, and some anesthetics potentially able to activate specific sleep-active regions.[12]
Two non-exclusionary mechanisms include membrane-mediated and direct protein-mediated anesthesia. Potential protein-mediated molecular targets are GABAA,and NMDA glutamate receptors. General anesthesia was hypothesized to either enhance the inhibitory transmission or reduce the excitatory transmission of neuro signaling.[13] Most volatile anesthetics have been found to be a GABAA agonist, although the site of action on the receptor remains unknown.[14] Ketamine is a non-competitive NMDA receptor antagonist.[15]
The chemical structure and properties of anesthetics, as first noted by Meyer and Overton, suggest they could target the plasma membrane. A membrane-mediated mechanism that could account for the activation of an ion channel remained elusive until recently. A study from 2020 demonstrated that inhaled anesthetics (chloroform and isoflurane) could displace phospholipase D2 from ordered lipid domains in the plasma membrane, which led to the production of the signaling molecule phosphatidic acid (PA). The signaling molecule activated TWIK-related K+ channels (TREK-1), a channel involved in anesthesia. PLDnull fruit flies were shown to resist anesthesia, the results established a membrane mediated target for inhaled anesthetics.[16]
Preoperative evaluation
Prior to a planned procedure, the anesthesiologist reviews medical records, interviews the patient, and conducts a physical examination to obtain information regarding their medical history and current physical state, and to determine an appropriate anesthetic plan, including what combination of drugs and dosages will likely be needed for the patient's comfort and safety during the procedure. A variety of non-invasive and invasive monitoring devices may be necessary to ensure a safe and effective procedure. Key factors in this evaluation are the patient's age, gender, body mass index, medical and surgical history, current medications, exercise capacity, and fasting time.[17][18] Thorough and accurate preoperative evaluation is crucial for the effective safety of the anesthetic plan. For example, a patient who consumes significant quantities of alcohol or illicit drugs could be undermedicated during the procedure if they fail to disclose this fact, and this could lead to anaesthesia awareness or intraoperative hypertension.[2][19] Commonly used medications can also interact with anaesthetics, and failure to disclose such usage can increase the risk during the operation. Inaccurate timing of last meal can also increase the risk for aspiration of food, and lead to serious complications.[6]
An important aspect of pre-anaesthetic evaluation is an assessment of the patient's airway, involving inspection of the mouth opening and visualisation of the soft tissues of the pharynx.[20] The condition of teeth and location of dental crowns are checked, and neck flexibility and head extension are observed.[21][22] The most commonly performed airway assessment is the Mallampati classification, which evaluates the airway base on the ability to view airway structures with the mouth open and the tongue protruding. Mallampati tests alone have limited accuracy, and other evaluations are routinely performed addition to the Mallampati test including mouth opening, thyromental distance, neck range of motion, and mandibular protrusion. In a patient with suspected distorted airway anatomy, endoscopy or ultrasound is sometimes used to evaluate the airway before planning for the airway management.[23]
Premedication
Prior to administration of a general anaesthetic, the anaesthetist may administer one or more drugs that complement or improve the quality or safety of the anaesthetic or simply provide anxiolysis. Premedication also often has mild sedative effects and may reduce the amount of anaesthetic agent required during the case.[6]
One commonly used premedication is clonidine, an alpha-2 adrenergic agonist.[24][25] It reduces postoperative shivering, postoperative nausea and vomiting, and emergence delirium.[6] However, a randomized controlled trial from 2021 demonstrated that clonidine is less effective at providing anxiolysis and more sedative in children of preschool age. Oral clonidine can take up to 45 minutes to take full effect,[26] The drawbacks of clonidine include hypotension and bradycardia, but these can be advantageous in patients with hypertension and tachycardia.[27] Another commonly used alpha-2 adrenergic agonist is dexmedetomidine, which is commonly used to provide a short term sedative effect (<24 hours). Dexmedetomidine and certain atypical antipsychotic agents may be also used in uncooperative children.[28]
Benzodiazepines are the most commonly used class of drugs for premedication. The most commonly utilized benzodiazepine is Midazolam, which is characterized by a rapid onset and short duration. Midazolam is effective in reducing preoperative anxiety, including separation anxiety in children.[29] It also provides mild sedation, sympathicolysis, and anterograde amnesia.[6]
Melatonin has been found to be effective as an anaesthetic premedication in both adults and children because of its hypnotic, anxiolytic, sedative, analgesic, and anticonvulsant properties. Recovery is more rapid after premedication with melatonin than with midazolam, and there is also a reduced incidence of post-operative agitation and delirium.[30] Melatonin has been shown to have a similar effect in reducing perioperative anxiety in adult patients compared to benzodiazepine.[31]
Another example of anaesthetic premedication is the preoperative administration of beta adrenergic antagonists, which reduce the burden of arrhythmias after cardiac surgery. However, evidence also has shown an association of increased adverse events with beta-blockers in non-cardiac surgery.[32] Anaesthesiologists may administer one or more antiemetic agents such as ondansetron, droperidol, or dexamethasone to prevent postoperative nausea and vomiting.[6] NSAIDs are commonly used analgesic premedication agent, and often reduce need for opioids such as fentanyl or sufentanil. Also gastrokinetic agents such as metoclopramide, and histamine antagonists such as famotidine.[6]
Non-pharmacologic preanaesthetic interventions include playing cognitive behavioral therapy, music therapy, aromatherapy, hypnosis massage, pre-operative preparation video, and guided imagery relaxation therapy, etc.[33] These techniques are particularly useful for children and patients with intellectual disabilities. Minimizing sensory stimulation or distraction by video games may help to reduce anxiety prior to or during induction of general anaesthesia. Larger high-quality studies are needed to confirm the most effective non-pharmacological approaches for reducing this type of anxiety.[34] Parental presence during premedication and induction of anaesthesia has not been shown to reduce anxiety in children.[34] It is suggested that parents who wish to attend should not be actively discouraged, and parents who prefer not to be present should not be actively encouraged to attend.[34]
Anesthesia and the brain
Anesthesia has little to no effect on brain function, unless there is an existing brain disruption. Barbiturates, or the drugs used to administer anesthesia do not effect auditory brain stem response.[35] An example of a brain disruption would be a concussion.[36] It can be risky and lead to further brain injury if anesthesia is used on a concussed person. Concussions create ionic shifts in the brain that adjust the neuronal transmembrane potential. In order to restore this potential more glucose has to be made to equal the potential that is lost. This can be very dangerous and lead to cell death. This makes the brain very vulnerable in surgery. There are also changes to cerebral blood flow. The injury complicates the oxygen blood flow and supply to the brain.
Stages of anaesthesia
Guedel's classification, described by Arthur Ernest Guedel in 1937,[3] describes four stages of anaesthesia. Despite newer anaesthetic agents and delivery techniques, which have led to more rapid onset of—and recovery from—anaesthesia (in some cases bypassing some of the stages entirely), the principles remain.
- Stage 1
- Stage 1, also known as induction, is the period between the administration of induction agents and loss of consciousness. During this stage, the patient progresses from analgesia without amnesia to analgesia with amnesia. Patients can carry on a conversation at this time, and may complain about visual disturbance.
- Stage 2
- Stage 2, also known as the excitement or delirium stage, is the period following loss of consciousness and marked by excited and delirious activity. During this stage, the patient's respiration and heart rate may become irregular. In addition, there may be uncontrolled movements, vomiting, suspension of breathing, and pupillary dilation. Because the combination of spastic movements, vomiting, and irregular respiration may compromise the patient's airway, rapidly acting drugs are used to minimize time in this stage and reach Stage 3 as fast as possible.
- Stage 3
- In Stage 3, also known as surgical anaesthesia, the skeletal muscles relax, vomiting stops. Respiratory depression and cessation of eye movements are the hallmarks of this stage. The patient is unconscious and ready for surgery. This stage is divided into four planes:
- The eyes roll, then become fixed; eyelid and swallow reflexes are lost. Still have regular spontaneous breathing;
- Corneal and laryngeal reflexes are lost;
- The pupillary light reflex is lost; and the process is marked by complete relaxation of abdominal and intercostal muscles. Ideal level of anesthesia for most surgeries.
- Full diaphragm paralysis and irregular shallow abdominal respiration occur.[37]
- Stage 4
- Stage 4, also known as overdose, occurs when too much anaesthetic medication is given relative to the amount of surgical stimulation and the patient has severe brainstem or medullary depression, resulting in a cessation of respiration and potential cardiovascular collapse. This stage is lethal without cardiovascular and respiratory support.[3]
Induction
General anaesthesia is usually induced in an operating theatre or in a dedicated anaesthetic room adjacent to the theatre. General anaesthesia may also be conducted in other locations, such as an endoscopy suite, intensive care unit, radiology or cardiology department, emergency department, ambulance, or at the site of a disaster where extrication of the patient may be impossible or impractical.
Anaesthetic agents may be administered by various routes, including inhalation, injection (intravenous, intramuscular, or subcutaneous), oral, and rectal. Once they enter the circulatory system, the agents are transported to their biochemical sites of action in the central and autonomic nervous systems.
Most general anaesthetics are induced either intravenously or by inhalation. Commonly used intravenous induction agents include propofol, sodium thiopental, etomidate, methohexital, and ketamine. Inhalational anaesthesia may be chosen when intravenous access is difficult to obtain (e.g., children), when difficulty maintaining the airway is anticipated, or when the patient prefers it. Sevoflurane is the most commonly used agent for inhalational induction, because it is less irritating to the tracheobronchial tree than other agents.[38]
As an example sequence of induction drugs:
- Pre-oxygenation or denitrogenation to fill lungs with 100% oxygen to permit a longer period of apnea during intubation without affecting blood oxygen levels
- Fentanyl for systemic analgesia during intubation
- Propofol for sedation for intubation
- Switching from oxygen to a mixture of oxygen and inhalational anesthetic once intubation is complete
Laryngoscopy and intubation are both very stimulating. The process of induction blunts the response to these maneuvers while simultaneously inducing a near-coma state to prevent awareness.
Physiologic monitoring
Several monitoring technologies allow for a controlled induction of, maintenance of, and emergence from general anaesthesia. Standard for basic anesthetic monitoring is a guideline published by the ASA, which describes that the patient's oxygenation, ventilation, circulation and temperature should be continually evaluated during anesthetic.[39]
- Continuous electrocardiography (ECG or EKG): Electrodes are placed on the patient's skin to monitor heart rate and rhythm. This may also help the anaesthesiologist to identify early signs of heart ischaemia. Typically lead II and V5 are monitored for arrhythmias and ischemia, respectively.
- Continuous pulse oximetry (SpO2): A device is placed, usually on a finger, to allow for early detection of a fall in a patient's hemoglobin saturation with oxygen (hypoxaemia).
- Blood pressure monitoring: There are two methods of measuring the patient's blood pressure. The first, and most common, is non-invasive blood pressure (NIBP) monitoring. This involves placing a blood pressure cuff around the patient's arm, forearm, or leg. A machine takes blood pressure readings at regular, preset intervals throughout the surgery. The second method is invasive blood pressure (IBP) monitoring, which allows beat to beat monitoring of blood pressure. This method is reserved for patients with significant heart or lung disease, the critically ill, and those undergoing major procedures such as cardiac or transplant surgery, or when large blood loss is expected. It involves placing a special type of plastic cannula in an artery, usually in the wrist (radial artery) or groin (femoral artery).
- Agent concentration measurement: anaesthetic machines typically have monitors to measure the percentage of inhalational anaesthetic agents used as well as exhalation concentrations. These monitors include measuring oxygen, carbon dioxide, and inhalational anaesthetics (e.g., nitrous oxide, isoflurane).
- Oxygen measurement: Almost all circuits have an alarm in case oxygen delivery to the patient is compromised. The alarm goes off if the fraction of inspired oxygen drops below a set threshold.
- A circuit disconnect alarm or low pressure alarm indicates failure of the circuit to achieve a given pressure during mechanical ventilation.
- Capnography measures the amount of carbon dioxide exhaled by the patient in percent or mmHg, allowing the anaesthesiologist to assess the adequacy of ventilation. MmHg is usually used to allow the provider to see more subtle changes.
- Temperature measurement to discern hypothermia or fever, and to allow early detection of malignant hyperthermia.
- Electroencephalography, entropy monitoring, or other systems may be used to verify the depth of anaesthesia. This reduces the likelihood of anaesthesia awareness and of overdose.
Airway management
Anaesthetized patients lose protective airway reflexes (such as coughing), airway patency, and sometimes a regular breathing pattern due to the effects of anaesthetics, opioids, or muscle relaxants. To maintain an open airway and regulate breathing, some form of breathing tube is inserted after the patient is unconscious. To enable mechanical ventilation, an endotracheal tube is often used, although there are alternative devices that can assist respiration, such as face masks or laryngeal mask airways. Generally, full mechanical ventilation is only used if a very deep state of general anaesthesia is to be induced for a major procedure, and/or with a profoundly ill or injured patient. That said, induction of general anaesthesia usually results in apnea and requires ventilation until the drugs wear off and spontaneous breathing starts. In other words, ventilation may be required for both induction and maintenance of general anaesthesia or just during the induction. However, mechanical ventilation can provide ventilatory support during spontaneous breathing to ensure adequate gas exchange.
General anaesthesia can also be induced with the patient spontaneously breathing and therefore maintaining their own oxygenation which can be beneficial in certain scenarios (e.g. difficult airway or tubeless surgery). Spontaneous ventilation has been traditionally maintained with inhalational agents (i.e. halothane or sevoflurane) which is called a gas or inhalational induction. Spontaneous ventilation can also be maintained using intravenous anaesthesia (e.g. propofol). Intravenous anaesthesia to maintain spontaneous respiration has certain advantages over inhalational agents (i.e. suppressed laryngeal reflexes) however it requires careful titration. Spontaneous Respiration using Intravenous anaesthesia and High-flow nasal oxygen (STRIVE Hi) is a technique that has been used in difficult and obstructed airways.[40]
Eye management
General anaesthesia reduces the tonic contraction of the orbicularis oculi muscle, causing lagophthalmos (incomplete eye closure) in 59% of people.[41] In addition, tear production and tear-film stability are reduced, resulting in corneal epithelial drying and reduced lysosomal protection. The protection afforded by Bell's phenomenon (in which the eyeball turns upward during sleep, protecting the cornea) is also lost. Careful management is required to reduce the likelihood of eye injuries during general anaesthesia.[42] Some of the methods to prevent eye injury during general anesthesia includes taping the eyelids shut, use of eye ointments, and specially designed eye protective goggles.
Neuromuscular blockade
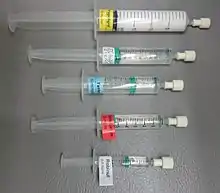
- Propofol, a hypnotic
- Ephedrine, in case of hypotension
- Fentanyl, for analgesia
- Atracurium, for neuromuscular block
- Glycopyrronium bromide (here under trade name Robinul), reducing secretions
Paralysis, or temporary muscle relaxation with a neuromuscular blocker, is an integral part of modern anaesthesia. The first drug used for this purpose was curare, introduced in the 1940s, which has now been superseded by drugs with fewer side effects and, generally, shorter duration of action.[43] Muscle relaxation allows surgery within major body cavities, such as the abdomen and thorax, without the need for very deep anaesthesia, and also facilitates endotracheal intubation.
Acetylcholine, a natural neurotransmitter found at the neuromuscular junction, causes muscles to contract when it is released from nerve endings. Muscle paralytic drugs work by preventing acetylcholine from attaching to its receptor. Paralysis of the muscles of respiration—the diaphragm and intercostal muscles of the chest—requires that some form of artificial respiration be implemented. Because the muscles of the larynx are also paralysed, the airway usually needs to be protected by means of an endotracheal tube.[6]
Paralysis is most easily monitored by means of a peripheral nerve stimulator. This device intermittently sends short electrical pulses through the skin over a peripheral nerve while the contraction of a muscle supplied by that nerve is observed. The effects of muscle relaxants are commonly reversed at the end of surgery by anticholinesterase drugs, which are administered in combination with muscarinic anticholinergic drugs to minimize side effects. Examples of skeletal muscle relaxants in use today are pancuronium, rocuronium, vecuronium, cisatracurium, atracurium, mivacurium, and succinylcholine.Novel neuromuscular blockade reversal agents such as sugammadex may also be used; it works by directly binding muscle relaxants and removing it from the neuromuscular junction. Sugammadex was approved for use in the United States in 2015, and rapidly gained popularity. A study from 2022 has shown that Sugammadex and neostigmine are likely similarly safe in the reversal of neuromuscular blockade.[44]
Maintenance
The duration of action of intravenous induction agents is generally 5 to 10 minutes, after which spontaneous recovery of consciousness will occur.[45] In order to prolong unconsciousness for the duration of surgery, anaesthesia must be maintained. This is achieved by allowing the patient to breathe a carefully controlled mixture of oxygen and a volatile anaesthetic agent, or by administering intravenous medication (usually propofol). Inhaled anaesthetic agents are also frequently supplemented by intravenous analgesic agents, such as opioids (usually fentanyl or a fentanyl derivative) and sedatives (usually propofol or midazolam). Propofol can be used for total intravenous anaesthetia (TIVA), therefore supplementation by inhalation agents is not required.[46] General anesthesia is usually considered safe; however, there are reported cases of patients with distortion of taste and/or smell due to local anesthetics, stroke, nerve damage, or as a side effect of general anesthesia.[47][48]
At the end of surgery, administration of anaesthetic agents is discontinued. Recovery of consciousness occurs when the concentration of anaesthetic in the brain drops below a certain level (this occurs usually within 1 to 30 minutes, mostly depending on the duration of surgery).[6]
In the 1990s, a novel method of maintaining anaesthesia was developed in Glasgow, Scotland. Called target controlled infusion (TCI), it involves using a computer-controlled syringe driver (pump) to infuse propofol throughout the duration of surgery, removing the need for a volatile anaesthetic and allowing pharmacologic principles to more precisely guide the amount of the drug used by setting the desired drug concentration. Advantages include faster recovery from anaesthesia, reduced incidence of postoperative nausea and vomiting, and absence of a trigger for malignant hyperthermia. At present, TCI is not permitted in the United States, but a syringe pump delivering a specific rate of medication is commonly used instead.[49]
Other medications are occasionally used to treat side effects or prevent complications. They include antihypertensives to treat high blood pressure; ephedrine or phenylephrine to treat low blood pressure; salbutamol to treat asthma, laryngospasm, or bronchospasm; and epinephrine or diphenhydramine to treat allergic reactions. Glucocorticoids or antibiotics are sometimes given to prevent inflammation and infection, respectively.[6]
Emergence
Emergence is the return to baseline physiologic function of all organ systems after the cessation of general anaesthetics. This stage may be accompanied by temporary neurologic phenomena, such as agitated emergence (acute mental confusion), aphasia (impaired production or comprehension of speech), or focal impairment in sensory or motor function. Shivering is also fairly common and can be clinically significant because it causes an increase in oxygen consumption, carbon dioxide production, cardiac output, heart rate, and systemic blood pressure. The proposed mechanism is based on the observation that the spinal cord recovers at a faster rate than the brain. This results in uninhibited spinal reflexes manifested as clonic activity (shivering). This theory is supported by the fact that doxapram, a CNS stimulant, is somewhat effective in abolishing postoperative shivering.[50] Cardiovascular events such as increased or decreased blood pressure, rapid heart rate, or other cardiac dysrhythmias are also common during emergence from general anaesthesia, as are respiratory symptoms such as dyspnoea. Responding and following verbal command, is a criterion commonly utilized to assess the patient's readiness for tracheal extubation.[6]
Postoperative care

Postoperative pain is managed in the anaesthesia recovery unit (PACU) with regional analgesia or oral, transdermal, or parenteral medication. Patients may be given opioids, as well as other medications like non steroidal anti-inflammatory drugs and acetaminophen.[51] Sometimes, opioid medication is administered by the patient themselves using a system called a patient controlled analgesic.[52] The patient presses a button to activate a syringe device and receive a preset dose or "bolus" of the drug, usually a strong opioid such as morphine, fentanyl, or oxycodone (e.g., one milligram of morphine). The PCA device then "locks out" for a preset period to allow the drug to take effect, and also prevent the patient from overdosing. If the patient becomes too sleepy or sedated, he or she makes no more requests. This confers a fail-safe aspect that is lacking in continuous-infusion techniques. If these medications cannot effectively manage the pain, local anesthetic may be directly injected to the nerve in a procedure called a nerve block.[53][54]
In the recovery unit, many vital signs are monitored, including oxygen saturation,[55][56] heart rhythm and respiration,[55][57] blood pressure,[55] and core body temperature.
Postanesthetic shivering is common. Apart from causing discomfort and exacerbating pain, shivering has been shown to increase oxygen consumption, catecholamine release, risk for hypothermia, and induce lactic acidosis.[58] A number of techniques are used to reduce shivering, such as warm blankets,[59][60] or wrapping the patient in a sheet that circulates warmed air, called a bair hugger.[61][62] If the shivering cannot be managed with external warming devices, drugs such as dexmedetomidine,[63][64] or other α2-agonists, anticholinergics, central nervous system stimulants, or corticosteroids may be used.[51][65]
In many cases, opioids used in general anaesthesia can cause postoperative ileus, even after non-abdominal surgery. Administration of a μ-opioid antagonist such as alvimopan immediately after surgery can help accelerate the timing of hospital discharge, but does not reduce the development of paralytic ileus.[66]
Enhanced Recovery After Surgery (ERAS) is a society that provides up-to-date guidelines and consensus to ensure continuity of care and improve recovery and peri-operative care. Adherence to the pathway and guidelines has been shown to associate with improved post-operative outcomes and lower costs to the health care system.[67]
Perioperative mortality
Most perioperative mortality is attributable to complications from the operation, such as haemorrhage, sepsis, and failure of vital organs. Over the last several decades, the overall anesthesia related mortality rate improved significantly for anesthetics administered. Advancements in monitoring equipment, anesthetic agents, and increased focus on perioperative safety are some reasons for the decrease in perioperative mortality. In the United States, the current estimated anesthesia-related mortality is about 1.1 per million population per year. The highest death rates were found in the geriatric population, especially those 85 and older.[68] A review from 2018 examined perioperative anesthesia interventions and their impact on anesthesia-related mortality. Interventions found to reduce mortality include pharmacotherapy, ventilation, transfusion, nutrition, glucose control, dialysis and medical device.[69] Interestingly, a randomized controlled trial from 2022 demonstrated that there is no significant difference in mortality between patient receiving handover from one clinician to another compared to the control group.[70]
Mortality directly related to anaesthetic management is very uncommon but may be caused by pulmonary aspiration of gastric contents,[71] asphyxiation,[72] or anaphylaxis.[4] These in turn may result from malfunction of anaesthesia-related equipment or, more commonly, human error. In 1984, after a television programme highlighting anaesthesia mishaps aired in the United States, American anaesthesiologist Ellison C. Pierce appointed the Anesthesia Patient Safety and Risk Management Committee within the American Society of Anesthesiologists.[73] This committee was tasked with determining and reducing the causes of anaesthesia-related morbidity and mortality.[73] An outgrowth of this committee, the Anesthesia Patient Safety Foundation, was created in 1985 as an independent, nonprofit corporation with the goal "that no patient shall be harmed by anesthesia".[74]
The rare but major complication of general anaesthesia is malignant hyperthermia.[75][76] All major hospital should have protocol in place with emergence drug cart near the OR for this potential complication.[77]
See also
References
- 1 2 Takla, A; Savulescu, J; Wilkinson, DJC; Pandit, JJ (October 2021). "General anaesthesia in end-of-life care: extending the indications for anaesthesia beyond surgery". Anaesthesia. 76 (10): 1308–1315. doi:10.1111/anae.15459. PMID 33878803.
- 1 2 Budworth L, Prestwich A, Lawton R, Kotzé A, Kellar I (4 February 2019). "Preoperative Interventions for Alcohol and Other Recreational Substance Use: A Systematic Review and Meta-Analysis". Frontiers in Psychology. 10: 34. doi:10.3389/fpsyg.2019.00034. PMC 6369879. PMID 30778307.
- 1 2 3 4 Hewer CL (August 1937). "The Stages and Signs of General Anaesthesia". British Medical Journal. 2 (3996): 274–276. doi:10.1136/bmj.2.3996.274. PMC 2087073. PMID 20780832.
- 1 2 Dewachter P, Mouton-Faivre C, Emala CW (November 2009). "Anaphylaxis and anesthesia: controversies and new insights". Anesthesiology. 111 (5): 1141–1150. doi:10.1097/ALN.0b013e3181bbd443. PMID 19858877.
- 1 2 "Position on Monitored Anesthesia Care" (PDF). American Society of Anesthesiologists. Archived from the original (PDF) on 11 November 2022. Retrieved 6 November 2022.
Approved by the House of Delegates on October 25, 2005, and last amended on October 17, 2018
- 1 2 3 4 5 6 7 8 9 10 11 Michael A. Gropper, ed. (2020). Miller's anesthesia (Ninth ed.). Philadelphia, PA: Elsevier. ISBN 978-0-323-61264-7. OCLC 1124935549.
- ↑ Toledo-Pereyra LH (June 2015). "Medical Renaissance". Journal of Investigative Surgery. 28 (3): 127–130. doi:10.3109/08941939.2015.1054747. PMID 26065591. S2CID 207482973.
- ↑ Schlich T (July 2012). "Asepsis and bacteriology: a realignment of surgery and laboratory science". Medical History. 56 (3): 308–334. doi:10.1017/mdh.2012.22. PMC 3426977. PMID 23002302.
- ↑ Dote K, Ikemune K, Desaki Y, Nandate H, Konisi A, Yorozuya T, Makino H (January 2017). "Two Japanese Pioneers in Anesthesiology: Seishū Hanaoka and Gendai Kamada". Journal of Anesthesia History. 3 (1): 19–23. doi:10.1016/j.janh.2016.12.002. PMID 28160985.
- ↑ Jevtovic-Todorovic V (September 2016). "General Anesthetics and Neurotoxicity: How Much Do We Know?". Anesthesiology Clinics. 34 (3): 439–451. doi:10.1016/j.anclin.2016.04.001. PMC 5477636. PMID 27521190.
- ↑ Frazier J (26 January 2018). "Plants, Like People, Succumb to Anesthesia". Scientific American. Retrieved 26 January 2018.
- ↑ Moody, Olivia A.; Zhang, Edlyn R.; Vincent, Kathleen F.; Kato, Risako; Melonakos, Eric D.; Nehs, Christa J.; Solt, Ken (1 May 2021). "The Neural Circuits Underlying General Anesthesia and Sleep". Anesthesia and Analgesia. 132 (5): 1254–1264. doi:10.1213/ANE.0000000000005361. ISSN 1526-7598. PMC 8054915. PMID 33857967.
- ↑ Lambert, David G. (1 May 2020). "Mechanisms of action of general anaesthetic drugs". Anaesthesia & Intensive Care Medicine. 21 (5): 235–237. doi:10.1016/j.mpaic.2020.02.006. ISSN 1472-0299.
- ↑ Woll, Kellie A.; Zhou, Xiaojuan; Bhanu, Natarajan V.; Garcia, Benjamin A.; Covarrubias, Manuel; Miller, Keith W.; Eckenhoff, Roderic G. (August 2018). "Identification of binding sites contributing to volatile anesthetic effects on GABA type A receptors". The FASEB Journal. 32 (8): 4172–4189. doi:10.1096/fj.201701347R. ISSN 0892-6638. PMC 6044061. PMID 29505303.
- ↑ Zhang, Youyi; Ye, Fei; Zhang, Tongtong; Lv, Shiyun; Zhou, Liping; Du, Daohai; Lin, He; Guo, Fei; Luo, Cheng; Zhu, Shujia (August 2021). "Structural basis of ketamine action on human NMDA receptors". Nature. 596 (7871): 301–305. Bibcode:2021Natur.596..301Z. doi:10.1038/s41586-021-03769-9. ISSN 1476-4687. PMID 34321660. S2CID 236496390.
- ↑ Pavel MA, Petersen EN, Wang H, Lerner RA, Hansen SB (June 2020). "Studies on the mechanism of general anesthesia". Proceedings of the National Academy of Sciences of the United States of America. 117 (24): 13757–13766. Bibcode:2020PNAS..11713757P. doi:10.1073/pnas.2004259117. PMC 7306821. PMID 32467161.
- ↑ Lederman D, Easwar J, Feldman J, Shapiro V (August 2019). "Anesthetic considerations for lung resection: preoperative assessment, intraoperative challenges and postoperative analgesia". Annals of Translational Medicine. 7 (15): 356. doi:10.21037/atm.2019.03.67. PMC 6712248. PMID 31516902.
- ↑ Izumo W, Higuchi R, Yazawa T, Uemura S, Shiihara M, Yamamoto M (December 2019). "Evaluation of preoperative risk factors for postpancreatectomy hemorrhage". Langenbeck's Archives of Surgery. 404 (8): 967–974. doi:10.1007/s00423-019-01830-w. PMC 6935390. PMID 31650216.
- ↑ Siriphuwanun V, Punjasawadwong Y, Saengyo S, Rerkasem K (18 October 2018). "Incidences and factors associated with perioperative cardiac arrest in trauma patients receiving anesthesia". Risk Management and Healthcare Policy. 11: 177–187. doi:10.2147/rmhp.s178950. PMC 6201994. PMID 30425598.
- ↑ Mushambi MC, Jaladi S (June 2016). "Airway management and training in obstetric anaesthesia". Current Opinion in Anesthesiology. 29 (3): 261–267. doi:10.1097/ACO.0000000000000309. PMID 26844863. S2CID 27527932.
- ↑ Rehak A, Watterson LM (June 2020). "Institutional preparedness to prevent and manage anaesthesia-related 'can't intubate, can't oxygenate' events in Australian and New Zealand teaching hospitals". Anaesthesia. 75 (6): 767–774. doi:10.1111/anae.14909. PMID 31709522. S2CID 207944753.
- ↑ Schieren M, Kleinschmidt J, Schmutz A, Loop T, Staat M, Gatzweiler KH, et al. (December 2019). "Comparison of forces acting on maxillary incisors during tracheal intubation with different laryngoscopy techniques: a blinded manikin study". Anaesthesia. 74 (12): 1563–1571. doi:10.1111/anae.14815. PMID 31448404.
- ↑ Roth, Dominik; Pace, Nathan L.; Lee, Anna; Hovhannisyan, Karen; Warenits, Alexandra-Maria; Arrich, Jasmin; Herkner, Harald (15 May 2018). "Airway physical examination tests for detection of difficult airway management in apparently normal adult patients". The Cochrane Database of Systematic Reviews. 5 (5): CD008874. doi:10.1002/14651858.CD008874.pub2. ISSN 1469-493X. PMC 6404686. PMID 29761867.
- ↑ Bergendahl H, Lönnqvist PA, Eksborg S (February 2006). "Clonidine in paediatric anaesthesia: review of the literature and comparison with benzodiazepines for premedication". Acta Anaesthesiologica Scandinavica. 50 (2): 135–143. doi:10.1111/j.1399-6576.2006.00940.x. PMID 16430532. S2CID 25797363. Archived from the original on 16 December 2012.
- ↑ Dahmani S, Brasher C, Stany I, Golmard J, Skhiri A, Bruneau B, et al. (April 2010). "Premedication with clonidine is superior to benzodiazepines. A meta analysis of published studies". Acta Anaesthesiologica Scandinavica. 54 (4): 397–402. doi:10.1111/j.1399-6576.2009.02207.x. PMID 20085541. S2CID 205430269.
- ↑ Bromfalk, Åsa; Myrberg, Tomi; Walldén, Jakob; Engström, Åsa; Hultin, Magnus (November 2021). Cravero, Joseph (ed.). "Preoperative anxiety in preschool children: A randomized clinical trial comparing midazolam, clonidine, and dexmedetomidine". Pediatric Anesthesia. 31 (11): 1225–1233. doi:10.1111/pan.14279. ISSN 1155-5645. PMID 34403548. S2CID 237197251.
- ↑ Henry RG, Raybould TP, Romond K, Kouzoukas DE, Challman SD (March 2018). "Clonidine as a preoperative sedative". Special Care in Dentistry. 38 (2): 80–88. doi:10.1111/scd.12269. PMID 29364538. S2CID 3875130.
- ↑ Manning, Alexander N.; Bezzo, Leah K.; Hobson, Jamie K.; Zoeller, Justine E.; Brown, Courtney A.; Henderson, Kristin J. (October 2020). "Dexmedetomidine Dosing to Prevent Pediatric Emergence Delirium". AANA Journal. 88 (5): 359–364. ISSN 2162-5239. PMID 32990204.
- ↑ El Batawi, Hisham Yehia (2015). "Effect of preoperative oral midazolam sedation on separation anxiety and emergence delirium among children undergoing dental treatment under general anesthesia". Journal of International Society of Preventive & Community Dentistry. 5 (2): 88–94. doi:10.4103/2231-0762.155728. ISSN 2231-0762. PMC 4415335. PMID 25992332.
- ↑ Naguib M, Gottumukkala V, Goldstein PA (January 2007). "Melatonin and anesthesia: a clinical perspective". Journal of Pineal Research. 42 (1): 12–21. doi:10.1111/j.1600-079X.2006.00384.x. PMID 17198534.
- ↑ Madsen BK, Zetner D, Møller AM, Rosenberg J (December 2020). "Melatonin for preoperative and postoperative anxiety in adults". The Cochrane Database of Systematic Reviews. 2020 (12): CD009861. doi:10.1002/14651858.CD009861.pub3. PMC 8092422. PMID 33319916.
- ↑ Blessberger H, Kammler J, Domanovits H, Schlager O, Wildner B, Azar D, et al. (Cochrane Anaesthesia Group) (March 2018). "Perioperative beta-blockers for preventing surgery-related mortality and morbidity". The Cochrane Database of Systematic Reviews. 2018 (3): CD004476. doi:10.1002/14651858.CD004476.pub3. PMC 6494407. PMID 29533470.
- ↑ Wang, Rulin; Huang, Xin; Wang, Yuan; Akbari, Masod (11 April 2022). "Non-pharmacologic Approaches in Preoperative Anxiety, a Comprehensive Review". Frontiers in Public Health. 10: 854673. doi:10.3389/fpubh.2022.854673. ISSN 2296-2565. PMC 9035831. PMID 35480569.
- 1 2 3 Manyande A, Cyna AM, Yip P, Chooi C, Middleton P (July 2015). "Non-pharmacological interventions for assisting the induction of anaesthesia in children". The Cochrane Database of Systematic Reviews. 2015 (7): CD006447. doi:10.1002/14651858.CD006447.pub3. PMC 8935979. PMID 26171895.
- ↑ Smith, D.I.; Mills, J.H. (May 1989). "Anesthesia effects: auditory brain-stem response". Electroencephalography and Clinical Neurophysiology. 72 (5): 422–428. doi:10.1016/0013-4694(89)90047-3. ISSN 0013-4694. PMID 2469566.
- ↑ Rasouli, Mohammed R.; Kavin, Michelle; Stache, Stephen; Mahla, Michael E.; Schwenk, Eric S. (February 2020). "Anesthesia for the patient with a recently diagnosed concussion: think about the brain!". Korean Journal of Anesthesiology. 73 (1): 3–7. doi:10.4097/kja.19272. ISSN 2005-7563. PMC 7000285. PMID 31257815.
- ↑ Hedenstierna G, Edmark L (September 2015). "Effects of anesthesia on the respiratory system". Best Practice & Research. Clinical Anaesthesiology. 29 (3): 273–284. doi:10.1016/j.bpa.2015.08.008. PMID 26643094.
- ↑ Ebert TJ, Muzi M, Lopatka CW (July 1995). "Neurocirculatory responses to sevoflurane in humans. A comparison to desflurane". Anesthesiology. 83 (1): 88–95. doi:10.1097/00000542-199507000-00011. PMID 7605024. S2CID 24335733.
- ↑ "Standards for Basic Anesthetic Monitoring" (PDF). American Society of Anesthesiologists. Retrieved 10 November 2022.
Approved by the ASA House of Delegates on October 21, 1986, last amended on October 20, 2010, and reaffirmed on December 13, 2020
- ↑ Booth AW, Vidhani K, Lee PK, Thomsett CM (March 2017). "SponTaneous Respiration using IntraVEnous anaesthesia and Hi-flow nasal oxygen (STRIVE Hi) maintains oxygenation and airway patency during management of the obstructed airway: an observational study". British Journal of Anaesthesia. 118 (3): 444–451. doi:10.1093/bja/aew468. PMC 5409133. PMID 28203745.
- ↑ Contractor S, Hardman JG (2006). "Injury during anaesthesia". Continuing Education in Anaesthesia, Critical Care & Pain. 6 (2): 67–70. doi:10.1093/bjaceaccp/mkl004.
- ↑ Nair PN, White E (2014). "Care of the eye during anaesthesia and intensive care". Anaesthesia & Intensive Care Medicine. 15: 40–43. doi:10.1016/j.mpaic.2013.11.008.
- ↑ Eger, Edmond I II; Saidman, Lawrence J.; Westhorpe, Rod N., eds. (2014). The Wondrous Story of Anaesthesia. Springer. p. 438. ISBN 978-1-4614-8440-0
- ↑ Ruetzler K, Li K, Chhabada S, Maheshwari K, Chahar P, Khanna S, et al. (May 2022). "Sugammadex Versus Neostigmine for Reversal of Residual Neuromuscular Blocks After Surgery: A Retrospective Cohort Analysis of Postoperative Side Effects". Anesthesia and Analgesia. 134 (5): 1043–1053. doi:10.1213/ANE.0000000000005842. PMID 35020636. S2CID 245907059.
- ↑ Khan KS, Hayes I, Buggy DJ (June 2014). "Pharmacology of anaesthetic agents I: intravenous anaesthetic agents". Continuing Education in Anaesthesia Critical Care & Pain. 14 (3): 100–105. doi:10.1093/bjaceaccp/mkt039. ISSN 1743-1816.
- ↑ Nimmo AF, Absalom AR, Bagshaw O, Biswas A, Cook TM, Costello A, et al. (February 2019). "Guidelines for the safe practice of total intravenous anaesthesia (TIVA): Joint Guidelines from the Association of Anaesthetists and the Society for Intravenous Anaesthesia". Anaesthesia. 74 (2): 211–224. doi:10.1111/anae.14428. PMID 30378102. S2CID 53107969.
- ↑ Baker JJ, Öberg S, Rosenberg J (December 2017). "Loss of Smell and Taste After General Anesthesia: A Case Report". A&A Case Reports. 9 (12): 346–348. doi:10.1213/XAA.0000000000000612. PMID 28767470.
- ↑ Elterman KG, Mallampati SR, Kaye AD, Urman RDPostoperative alterations in taste and smell. Anesth Pain Med. 2014;4:e18527
- ↑ Absalom, Anthony R.; Glen, John Iain B.; Zwart, Gerrit J. C.; Schnider, Thomas W.; Struys, Michel M. R. F. (January 2016). "Target-Controlled Infusion: A Mature Technology". Anesthesia and Analgesia. 122 (1): 70–78. doi:10.1213/ANE.0000000000001009. ISSN 1526-7598. PMID 26516798. S2CID 41023659.
- ↑ Basics of Anesthesia, 5th Edition Authors: Robert K. Stoelting & Ronald D. Miller ISBN 978-0-443-06801-0
- 1 2 Lopez MB (April 2018). "Postanaesthetic shivering - from pathophysiology to prevention". Romanian Journal of Anaesthesia and Intensive Care. 25 (1): 73–81. doi:10.21454/rjaic.7518.251.xum. PMC 5931188. PMID 29756066.
- ↑ Rajpal S, Gordon DB, Pellino TA, Strayer AL, Brost D, Trost GR, et al. (April 2010). "Comparison of perioperative oral multimodal analgesia versus IV PCA for spine surgery". Journal of Spinal Disorders & Techniques. 23 (2): 139–145. doi:10.1097/BSD.0b013e3181cf07ee. PMID 20375829. S2CID 5319313.
- ↑ Schnabel A, Reichl SU, Weibel S, Zahn PK, Kranke P, Pogatzki-Zahn E, Meyer-Frießem CH, et al. (Cochrane Anaesthesia Group) (October 2019). "Adductor canal blocks for postoperative pain treatment in adults undergoing knee surgery". The Cochrane Database of Systematic Reviews. 2019 (10). doi:10.1002/14651858.CD012262.pub2. PMC 6814953. PMID 31684698.
- ↑ Sharma A, Goel AD, Sharma PP, Vyas V, Agrawal SP (October 2019). "The Effect of Transversus Abdominis Plane Block for Analgesia in Patients Undergoing Liver Transplantation: A Systematic Review and Meta-Analysis". Turkish Journal of Anaesthesiology and Reanimation. 47 (5): 359–366. doi:10.5152/tjar.2019.60251. PMC 6756312. PMID 31572985.
- 1 2 3 Olsen RM, Aasvang EK, Meyhoff CS, Dissing Sorensen HB (October 2018). "Towards an automated multimodal clinical decision support system at the post anesthesia care unit". Computers in Biology and Medicine. 101: 15–21. doi:10.1016/j.compbiomed.2018.07.018. PMID 30092398. S2CID 51955389.
- ↑ Petersen C, Wetterslev J, Meyhoff CS (August 2018). "Perioperative hyperoxia and post-operative cardiac complications in adults undergoing non-cardiac surgery: Systematic review protocol". Acta Anaesthesiologica Scandinavica. 62 (7): 1014–1019. doi:10.1111/aas.13123. PMID 29664117.
- ↑ Orbach-Zinger S, Bizman I, Firman S, Lev S, Gat R, Ashwal E, et al. (October 2019). "Perioperative noninvasive cardiac output monitoring in parturients undergoing cesarean delivery with spinal anesthesia and prophylactic phenylephrine drip: a prospective observational cohort study". The Journal of Maternal-Fetal & Neonatal Medicine. 32 (19): 3153–3159. doi:10.1080/14767058.2018.1458835. PMID 29683007. S2CID 5039625.
- ↑ Lopez, Maria Bermudez (April 2018). "Postanaesthetic shivering – from pathophysiology to prevention". Romanian Journal of Anaesthesia and Intensive Care. 25 (1): 73–81. doi:10.21454/rjaic.7518.251.xum. ISSN 2392-7518. PMC 5931188. PMID 29756066.
- ↑ Shaw CA, Steelman VM, DeBerg J, Schweizer ML (May 2017). "Effectiveness of active and passive warming for the prevention of inadvertent hypothermia in patients receiving neuraxial anesthesia: A systematic review and meta-analysis of randomized controlled trials". Journal of Clinical Anesthesia. 38: 93–104. doi:10.1016/j.jclinane.2017.01.005. PMC 5381733. PMID 28372696.
- ↑ Alderson P, Campbell G, Smith AF, Warttig S, Nicholson A, Lewis SR, et al. (Cochrane Anaesthesia, Critical and Emergency Care Group) (June 2014). "Thermal insulation for preventing inadvertent perioperative hypothermia". The Cochrane Database of Systematic Reviews (6): CD009908. doi:10.1002/14651858.CD009908.pub2. PMID 24895945.
- ↑ Stanger R, Colyvas K, Cassey JG, Robinson IA, Armstrong P (August 2009). "Predicting the efficacy of convection warming in anaesthetized children". British Journal of Anaesthesia. 103 (2): 275–282. doi:10.1093/bja/aep160. PMID 19541677.
- ↑ Wagner K, Swanson E, Raymond CJ, Smith CE (June 2008). "Comparison of two convective warming systems during major abdominal and orthopedic surgery". Canadian Journal of Anaesthesia. 55 (6): 358–363. doi:10.1007/BF03021491. PMID 18566199.
- ↑ Zhang J, Zhang X, Wang H, Zhou H, Tian T, Wu A (22 August 2017). "Dexmedetomidine as a neuraxial adjuvant for prevention of perioperative shivering: Meta-analysis of randomized controlled trials". PLOS ONE. 12 (8): e0183154. Bibcode:2017PLoSO..1283154Z. doi:10.1371/journal.pone.0183154. PMC 5567500. PMID 28829798.
- ↑ Zhang X, Wang D, Shi M, Luo Y (April 2017). "Efficacy and Safety of Dexmedetomidine as an Adjuvant in Epidural Analgesia and Anesthesia: A Systematic Review and Meta-analysis of Randomized Controlled Trials". Clinical Drug Investigation. 37 (4): 343–354. doi:10.1007/s40261-016-0477-9. PMID 27812971. S2CID 5512397.
- ↑ English W (2002). "Post-operative shivering, causes, prevention and treatment (letter)". Update in Anaesthesia (15). Archived from the original on 29 May 2011. Retrieved 8 September 2010.
- ↑ Wattchow D, Heitmann P, Smolilo D, Spencer NJ, Parker D, Hibberd T, et al. (May 2021). "Postoperative ileus-An ongoing conundrum". Neurogastroenterology and Motility. 33 (5): e14046. doi:10.1111/nmo.14046. PMID 33252179. S2CID 227235118.
- ↑ Baldini G (2022). Enhanced recovery protocols & optimization of perioperative outcomes. Butterworth IV J.F., & Mackey D.C., & Wasnick J.D.(Eds.), Morgan & Mikhail's Clinical Anesthesiology, 7e. McGraw Hill. https://accessmedicine-mhmedical-com.ezproxy.med.ucf.edu/content.aspx?bookid=3194§ionid=266524617
- ↑ Li, Guohua; Warner, Margaret; Lang, Barbara H.; Huang, Lin; Sun, Lena S. (April 2009). "Epidemiology of Anesthesia-related Mortality in the United States, 1999–2005". Anesthesiology. 110 (4): 759–765. doi:10.1097/aln.0b013e31819b5bdc. ISSN 0003-3022. PMC 2697561. PMID 19322941.
- ↑ Boet, Sylvain; Etherington, Cole; Nicola, David; Beck, Andrew; Bragg, Susan; Carrigan, Ian D.; Larrigan, Sarah; Mendonca, Cassandra T.; Miao, Isaac; Postonogova, Tatyana; Walker, Benjamin; De Wit, José; Mohamed, Karim; Balaa, Nadia; Lalu, Manoj Mathew (30 November 2018). "Anesthesia interventions that alter perioperative mortality: a scoping review". Systematic Reviews. 7 (1): 218. doi:10.1186/s13643-018-0863-x. ISSN 2046-4053. PMC 6267894. PMID 30497505.
- ↑ Meersch, Melanie; Weiss, Raphael; Küllmar, Mira; Bergmann, Lars; Thompson, Astrid; Griep, Leonore; Kusmierz, Desiree; Buchholz, Annika; Wolf, Alexander; Nowak, Hartmuth; Rahmel, Tim; Adamzik, Michael; Haaker, Jan Gerrit; Goettker, Carina; Gruendel, Matthias (28 June 2022). "Effect of Intraoperative Handovers of Anesthesia Care on Mortality, Readmission, or Postoperative Complications Among Adults: The HandiCAP Randomized Clinical Trial". JAMA. 327 (24): 2403–2412. doi:10.1001/jama.2022.9451. ISSN 1538-3598. PMC 9167439. PMID 35665794.
- ↑ Engelhardt T, Webster NR (September 1999). "Pulmonary aspiration of gastric contents in anaesthesia". British Journal of Anaesthesia. 83 (3): 453–460. doi:10.1093/bja/83.3.453. PMID 10655918.
- ↑ Parker RB (July 1956). "Maternal death from aspiration asphyxia". British Medical Journal. 2 (4983): 16–19. doi:10.1136/bmj.2.4983.16. PMC 2034767. PMID 13329366.
- 1 2 Guadagnino C (2000). "Improving anesthesia safety". Narberth, Pennsylvania: Physician's News Digest, Inc. Archived from the original on 15 August 2010. Retrieved 8 September 2010.
- ↑ Stoelting RK (2010). "Foundation History". Indianapolis, IN: Anesthesia Patient Safety Foundation. Retrieved 8 September 2010.
- ↑ Baldo BA, Rose MA (January 2020). "The anaesthetist, opioid analgesic drugs, and serotonin toxicity: a mechanistic and clinical review". British Journal of Anaesthesia. 124 (1): 44–62. doi:10.1016/j.bja.2019.08.010. PMID 31653394.
- ↑ Kim KS, Kriss RS, Tautz TJ (December 2019). "Malignant Hyperthermia: A Clinical Review". Advances in Anesthesia. 37: 35–51. doi:10.1016/j.aan.2019.08.003. PMID 31677658. S2CID 207899269.
- ↑ Pollock N, Langtont E, Stowell K, Simpson C, McDonnell N (August 2004). "Safe duration of postoperative monitoring for malignant hyperthermia susceptible patients". Anaesthesia and Intensive Care. 32 (4): 502–509. doi:10.1177/0310057X0403200407. PMID 15675210.
External links
- Chloroform: The molecular lifesaver An article at University of Bristol providing interesting facts about chloroform.
- Australian & New Zealand College of Anaesthetists Monitoring Standard
- Royal College of Anaesthetists Patient Information page