 | |
| Clinical data | |
|---|---|
| Trade names | Technescan Mag3 |
| License data | |
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| KEGG | |
Technetium (99mTc) mertiatide is a radiopharmaceutical medication used in nuclear medicine to image the kidneys.[1] It is a renal imaging agent that is given by intravenous injection.[1]
It was approved for medical use in the United States in June 1990.[2]
Medical uses
Technetium (99mTc) mertiatide is indicated for use in the diagnosis of congenital and acquired abnormalities, renal failure, urinary tract obstruction, and calculi.[1]
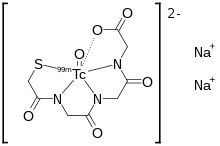
Chemistry
The active ingredient, betiatide, is reconstituted with sodium pertechnetate 99mTc injection to form technetium (99mTc) mertiatide.[1]
References
- 1 2 3 4 5 "Technescan Mag3- technescan tc 99m mertiatide injection, powder, lyophilized, for solution". DailyMed. 13 December 2022. Archived from the original on 2 February 2023. Retrieved 1 February 2023.
- ↑ "Technescan Mag3: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 13 December 2022. Retrieved 1 February 2023.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.