 | |
| Names | |
|---|---|
| Other names
1,4-Benzenedialdehyde, 1,4-Diformylbenzene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.009.805 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H6O2 | |
| Molar mass | 134.132 |
| Appearance | white to beige |
| Density | 1.06 g/mL |
| Melting point | 114–117 °C (237–243 °F; 387–390 K) |
| Boiling point | 245-248 |
| Hazards | |
| GHS labelling:[1] | |
  | |
| Danger | |
| H302, H311, H315, H319, H335 | |
| P261, P280, P304+P340, P305+P351+P338, P405, P501 | |
| Flash point | 76 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
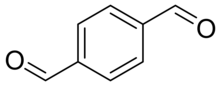
Terephthalaldehyde (TA) is an organic compound with the formula C6H4(CHO)2. It is one of three isomers of benzene dicarboxaldehyde, in which the aldehyde moieties are positioned in the para conformation on the benzene ring. Terephthalaldehyde appears as a white to beige solid, typically in the form of a powder. It is soluble in many organic solvents, such as alcohols (e.g., methanol or ethanol) and ethers (e.g., tetrahydrofuran or diethylether).
Preparation
Terepthalaldehyde can be synthesised from p-xylene in two steps.[2] First, p-xylene can be reacted with bromine to create α,α,α',α'-Tetrabromo-p-xylene. Next, sulphuric acid is introduced to create terephthaldehyde. Alternative procedures also describe the conversion of similar p-xylene derivatives into terephthalaldehyde.
Reactions and applications
Terphthalaldehyde is used in the preparation of imines, which are also commonly referred to as Schiff bases, following a condensation reaction with amines. During this reaction, water is also formed. This reaction is also reversible. However, due to aromatic conjugation with the benzene ring, the imines are relatively stable and will not easily hydrolyse back to the aldehyde. When in an acidic aqueous environment, the imines will start to hydrolyse more easily. Typically, an equilibrium between the imine and aldehyde is formed, which is dependent on the concentration of the relevant compounds and the pH of the solution.
Imines from terephthalaldehyde find use in the preparation of metal-organic coordination complexes. In addition, terepthaldehyde is a commonly used monomer in the production of imine polymers (polyimines),[3] covalent organic frameworks (COFs),[4] and molecular cages.[5] Terephthalaldehyde is also used as an intermediate for the preparation of dyes and fluorescent whitening agents.
Related compounds
References
- ↑ "Terephthalaldehyde". pubchem.ncbi.nlm.nih.gov.
- ↑ Snell, J. M.; Weissberger, A. (1940). "Terepthalaldehyde". Organic Syntheses. 20: 92. doi:10.15227/orgsyn.020.0092.
- ↑ Taynton, Philip; Zhu, Chengpu; Loob, Samuel; Schoemaker, Richard; Pritchard, James; Jin, Yinghua; Zhang, Wei (2016). "Re-healable polyimine thermosets: polymer composition and moisture sensitivity". Polymer Chemistry. 7 (46): 7052–7056. doi:10.1039/c6py01395c.
- ↑ Qu, Fei; Yan, Hang; Li, Kexin; You, JinMao; Han, Wenli (2020). "A covalent organic framework–MnO2 nanosheet system for determination of glutathione". Journal of Materials Science. 55 (23): 10022–10034. Bibcode:2020JMatS..5510022Q. doi:10.1007/s10853-020-04754-9. S2CID 218592879.
- ↑ Belowich, Matthew E.; Stoddart, J. Fraser (2012). "Dynamic imine chemistry". Chem. Soc. Rev. 41 (6): 2003–2024. doi:10.1039/C2CS15305J. PMID 22310886.