 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| H4O4Zn−2 | |
| Molar mass | 133.41 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
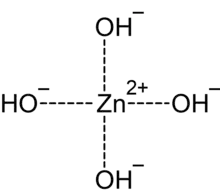
In chemistry, tetrahydroxozincate or tetrahydroxidozincate[1] is a divalent anion (negative ion) with formula Zn(OH)2−
4, with a central zinc atom in the +2 or (II) valence state coordinated to four hydroxide groups. It has Sp3 hybridization. It is the most common of the zincate anions, and is often called just zincate.
These names are also used for the salts containing that anion, such as sodium zincate Na2Zn(OH)4 [2] and calcium zincate CaZn(OH)4·2H2O [3]
Zincate salts can be obtained by reaction of zinc oxide (ZnO) or zinc hydroxide (Zn(OH)
2) and a strong base like sodium hydroxide.
It is now generally accepted that the resulting solutions contain the tetrahydroxozincate ion.[4] Earlier Raman studies had been interpreted as indicating the existence of linear ZnO2−
2 ions.[5]
Related anions and salts
The name "zincate" may also refer to a polymeric anion with formula approaching [Zn(OH)−
3]n, which forms salts such as NaZn(OH)
3·H
2O,[6] or to mixed oxides of zinc and less electronegative elements, such as Na
2ZnO
2.[7]
See also
- tetrachlorozincate or tetrachloridozincate, ZnCl2−
4 - tetranitratozincate, Zn(NO
3)2−
4
References
- ↑ IUPAC nomenclature of inorganic chemistry 2005
- ↑ Stahl, R.; Niewa, R.; Jacobs, H. (1999). "Synthese und Kristallstruktur von Na2Zn(OH)4". Zeitschrift für anorganische und allgemeine Chemie. 625: 48–50. doi:10.1002/(SICI)1521-3749(199901)625:1<48::AID-ZAAC48>3.0.CO;2-L.
- ↑ Sharma, Ram A. (1986). "Physico-Chemical Properties of Calcium Zincate". Journal of the Electrochemical Society. 133 (11): 2215–2219. Bibcode:1986JElS..133.2215S. doi:10.1149/1.2108376.
- ↑ Kaumudi I. Pandya; Andrea E. Russell; J. Mc Breen; W. E. O' Grady (1995). "EXAFS Investigations of Zn(II) in Concentrated Aqueous Hydroxide Solutions". The Journal of Physical Chemistry. 99 (31): 11967. doi:10.1021/j100031a026.
- ↑ Sharma, Shiv Kumar (1973). "Raman study of aqueous zinc oxide–alkali metal hydroxide system". The Journal of Chemical Physics. 58 (4): 1626–1629. Bibcode:1973JChPh..58.1626S. doi:10.1063/1.1679405.
- ↑ R. Stahl; H. Jacobs (1998). "Synthese und Kristallstruktur von NaZn(OH)3·H2O und NaZn(OH)3". Zeitschrift für anorganische und allgemeine Chemie. 624 (1): 25–29. doi:10.1002/(SICI)1521-3749(199801)624:1<25::AID-ZAAC25>3.0.CO;2-8.
- ↑ D. Trinschek; M. Jansen (1996). "Na2ZnO2, ein neues Natriumzinkat". Z. Naturforsch. 51 b: 711–4. doi:10.1515/znb-1996-0515. S2CID 96961116.