 | |
| Names | |
|---|---|
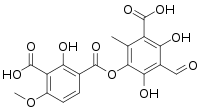
| IUPAC name
5-(3-carboxy-2-hydroxy-4-methoxy-6-methylbenzoyl)oxy-3-formyl-2,4-dihydroxy-6-methylbenzoic acid[1] | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C19H16O11 | |
| Molar mass | 420.326 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Thamnolic acid is a β-orcinol depside with the molecular formula C19H16O11.[2][3][1][4] Thamnolic acid was first isolated from the lichen Thamnolia vermicularis, but it also occur in Cladonia species.[5][6]
References
- 1 2 "Thamnolic acid". Pubchem.ncbi.NLM.nih.gov.
- ↑ Zeitschrift Für Naturforschung (in German). Dieterich'sche Verlagsbuchhandlung. 1967. p. 780.
- ↑ Elix, J. A.; Norfolk, S. (1975). "Synthesis of β-orcinol meta-depsides". Australian Journal of Chemistry. 28 (9): 2035–2041. doi:10.1071/ch9752035. ISSN 1445-0038.
- ↑ Bibliotheca lichenologica (in German). J. Cramer. 1973. p. 36. ISBN 978-3-443-58037-7.
- ↑ Der Stoffwechsel Sekundärer Pflanzenstoffe / The Metabolism of Secondary Plant Products. Springer Science & Business Media. 11 November 2013. p. 583. ISBN 978-3-662-26784-4.
- ↑ Brodo, Irwin M.; Sharnoff, Sylvia Duran; Sharnoff, Stephen; Nature, Canadian Museum of (1 January 2001). Lichens of North America. Yale University Press. p. 259. ISBN 978-0-300-08249-4.
Further reading
- Linskens, H. F.; Tracey, M. V.; Beiss, Ulrich; Bendall, Fay; Björk, Walter; Bohlmann, F.; Boman, Hans G.; Braun, Richard; Heinen, W.; Hesse, Manfred; Hofmann, Eduard; Hudson, J. R.; Knapp, Rüdiger; Lambert, Rudolf; Miller, Carlos O.; Pfleiderer, Gerhard; Sanwal, B. D.; Schmid, Hans; Shibata, Shoji; Stern, Herbert; Sucrow, Wolfgang; Tobiška, Josef; Zilliken, F. W. (13 March 2013). Modern Methods of Plant Analysis / Moderne Methoden der Pflanzenanalyse. Springer-Verlag. p. 184. ISBN 978-3-642-94878-7.
- Millot, M; Girardot, M; Dutreix, L; Imbert, C; Mambu, L (30 October 2014). "Lichen biodiversity: A source of secondary metabolites active against Candida biofilms". Planta Medica. 80 (16): s–0034–1394614. doi:10.1055/s-0034-1394614.
- Asahina, Yasuhiko; Hiraiwa, Mitio (5 February 1936). "Untersuchungen über Flechtenstoffe, LXIV. Mitteil.: Über die Konstitution der Thamnolsäure (IV. Mitteil.)". Berichte der Deutschen Chemischen Gesellschaft (A and B Series). 69 (2): 330–333. doi:10.1002/cber.19360690220.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.