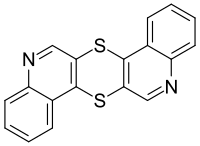
 | |
| Names | |
|---|---|
| IUPAC name
2,13-Dithia-10,21-diazapentacyclo[12.8.0.03,12.04,9.015,20]docosa-1(14),3(12),4,6,8,10,15,17,19,21-decaene | |
| Other names
Thiochinathren | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C18H10N2S2 | |
| Molar mass | 318.41 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Thioquinanthrene, also known as thiochinathren, is an aromatic organic chemical compound. It has the chemical formula C18H10N2S2 and reacts with alcoholates or alkoxides.[1] One of the key uses is to act as a catalyst poison in the Rosenmund reduction.[2][3] It has the IUPAC name of 2,13-dithia-10,21-diazapentacyclo[12.8.0.03,12.04,9.015,20]docosa-1(14),3(12),4,6,8,10,15,17,19,21-decaene.[4]
Rosenmund catalyst poison
In the Rosenmeund reaction, an acid chloride is reduced to an aldehyde. Continuing the reduction produces an alcohol. This further reaction is undesirable as the alcohol will now react with the acyl chloride to produce the unwanted ester product. For this reaction (over reduction) to be prevented, the catalyst needs to be poisoned. Thioquinanthrene was used initially, although other materials have been used since.[5][6][7][8]
References
- ↑ Maślankiewicz, Andrzej; Pluta, Krystian (1983-03-01). "Reactions of thioquinanthrene with alcoholates". Monatshefte für Chemie. 114 (3): 281–288. doi:10.1007/BF00798951. ISSN 1434-4475. S2CID 92762105.
- ↑ Dhaneeshwor (2021-02-21). "Rosenmund Reduction - Mechanism, Uses & Limitations". ProtonsTalk. Retrieved 2023-06-12.
- ↑ "Rosenmund Reduction Reaction: Catalyst, Mechanism & Uses". Collegedunia.com. 2022-12-22.
- ↑ PubChem. "Thiochinanthren". pubchem.ncbi.nlm.nih.gov. Retrieved 2023-06-12.
- ↑ "The Reduction of Aldehydes and Ketones". Chemistry LibreTexts. 2013-10-03. Retrieved 2023-06-12.
- ↑ Weygand, Conrad; Meusel, Werner (12 May 1943). "Über die Abstimmung der katalytischen Hydrierung, III. Mitteil.: Thioharnstoff als Spezifikator bei der Bildung von Benzaldehyd aus Benzoylchlorid". Berichte der Deutschen Chemischen Gesellschaft (A and B Series) (in German). 76 (5): 503–504. doi:10.1002/cber.19430760510.
- ↑ Rosenmund, K. W.; Zetzsche, F. (1921). "Über die Beeinflussung der Wirksamkeit von Katalysatoren, 1. bis 5". Chemische Berichte (in German). 54 (3): 425–437, 638–647, 1092–1098, 2033–2037, 2038–2042. doi:10.1002/cber.19210540310.
- ↑ Mosettig, E.; Mozingo, R. (1948). "The Rosenmund Reduction of Acid Chlorides to Aldehydes". Organic Reactions. 4: 362–377. doi:10.1002/0471264180.or004.07. ISBN 0471264180.