 | |
| Names | |
|---|---|
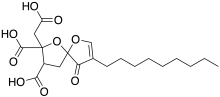
| IUPAC name
2-(Carboxymethyl)-8-nonyl-9-oxo-1,6-dioxaspiro[4.4]non-7-ene-2,3-dicarboxylic acid | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C20H28O9 | |
| Molar mass | 412.435 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Trachyspic acid is a fungal isolate that can inhibit heparanase.[1]
References
- ↑ Shiozawa H, Takahashi M, Takatsu T, Kinoshita T, Tanzawa K, Hosoya T, Furuya K, Takahashi S, Furihata K, Seto H (1995). "Trachyspic acid, a new metabolite produced by Talaromyces trachyspermus, that inhibits tumor cell heparanase: taxonomy of the producing strain, fermentation, isolation, structural elucidation, and biological activity". J Antibiot (Tokyo). 48 (5): 357–362. doi:10.7164/antibiotics.48.357. PMID 7797435.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.