 | |
 | |
| Names | |
|---|---|
| IUPAC name
Trimethylalumane | |
| Other names
Trimethylaluminum; aluminium trimethyl; aluminum trimethyl | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.000.776 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C6H18Al2 | |
| Molar mass | 144.17 g/mol 72.09 g/mol (C3H9Al) |
| Appearance | Colorless liquid |
| Density | 0.752 g/cm3 |
| Melting point | 15 °C (59 °F; 288 K) |
| Boiling point | 125–130 °C (257–266 °F; 398–403 K)[1][2] |
| Reacts | |
| Vapor pressure |
|
| Viscosity |
|
| Thermochemistry | |
Heat capacity (C) |
155.6 J/mol·K[2] |
Std molar entropy (S⦵298) |
209.4 J/mol·K[2] |
Std enthalpy of formation (ΔfH⦵298) |
−136.4 kJ/mol[2] |
Gibbs free energy (ΔfG⦵) |
−9.9 kJ/mol[2] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Pyrophoric |
| GHS labelling: | |
  [1] [1] | |
| Danger | |
| H250, H260, H314[1] | |
| P222, P223, P231+P232, P280, P370+P378, P422[1] | |
| NFPA 704 (fire diamond) | |
| Flash point | −17.0 °C (1.4 °F; 256.1 K)[1] |
| Related compounds | |
Related compounds |
Triethylaluminium |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Trimethylaluminium is one of the simplest examples of an organoaluminium compound. Despite its name it has the formula Al2(CH3)6 (abbreviated as Al2Me6 or TMA), as it exists as a dimer. This colorless liquid is pyrophoric. It is an industrially important compound, closely related to triethylaluminium.[3][4]
Structure and bonding
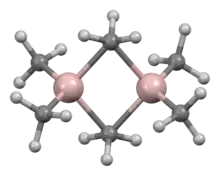
The structure and bonding in Al2R6 and diborane are analogous (R = alkyl). In Al2Me6, the Al-C(terminal) and Al-C(bridging) distances are 1.97 and 2.14 Å, respectively. The Al center is tetrahedral.[5] The carbon atoms of the bridging methyl groups are each surrounded by five neighbors: three hydrogen atoms and two aluminium atoms. The methyl groups interchange readily intramolecularly. At higher temperatures, the dimer cracks into monomeric AlMe3.[6]
Synthesis
TMA is prepared via a two-step process that can be summarized as follows:
- 2 Al + 6 CH3Cl + 6 Na → Al2(CH3)6 + 6 NaCl
Applications
Catalysis
Starting with the invention of Ziegler-Natta catalysis, organoaluminium compounds have a prominent role in the production of polyolefins, such as polyethylene and polypropylene. Methylaluminoxane, which is produced from TMA, is an activator for many transition metal catalysts.
Semiconductor applications
TMA is also used in semiconductor fabrication to deposit thin film, high-k dielectrics such as Al2O3 via the processes of chemical vapor deposition or atomic layer deposition. TMA is the preferred precursor for metalorganic vapour phase epitaxy (MOVPE) of aluminium-containing compound semiconductors, such as AlAs, AlN, AlP, AlSb, AlGaAs, AlInGaAs, AlInGaP, AlGaN, AlInGaN, AlInGaNP, etc. Criteria for TMA quality focus on (a) elemental impurities, (b) oxygenated and organic impurities.
Photovoltaic applications
In deposition processes very similar to semiconductor processing, TMA is used to deposit thin film, low-k (non-absorbing) dielectric layer stacks with Al2O3 via the processes of chemical vapor deposition or atomic layer deposition. The Al2O3 provides excellent surface passivation of p-doped silicon surfaces. The Al2O3 layer is typically the bottom layer with multiple silicon nitride (SixNy) layers for capping.
Reactions
Hydrolysis and related protonolysis reactions
Trimethylaluminium is hydrolyzed readily, even dangerously:
- Al2Me6 + 3 H2O → Al2O3 + 6 CH4
Under controlled conditions, the reaction can be stopped to give methylaluminoxane:
- AlMe3 + H2O → 1/n [AlMeO]n + 2 CH4
Alcoholysis and aminolysis reactions proceed comparably. For example, dimethylamine gives the dialuminium diamide dimer:[7]
- 2 AlMe3 + 2 HNMe2 → [AlMe2NMe2]2 + 2 CH4
Reactions with metal chlorides
TMA reacts with many metal halides to install alkyl groups. When combined with gallium trichloride, it gives trimethylgallium.[8] Al2Me6 reacts with aluminium trichloride to give (AlMe2Cl)2.
TMA/metal halide reactions have emerged as reagents in organic synthesis. Tebbe's reagent, which is used for the methylenation of esters and ketones, is prepared from TMA and titanocene dichloride.[9] In combination with 20 to 100 mol % Cp2ZrCl2 (zirconocene dichloride), the (CH3)2Al-CH3 adds "across" alkynes to give vinyl aluminium species that are useful in organic synthesis in a reaction known as carboalumination.[10]
Adducts
As for other "electron-deficient" compounds, trimethylaluminium gives adducts R3N.AlMe3. The Lewis acid properties of AlMe3 have been quantified.[11] The enthalpy data show that AlMe3 is a hard acid and its acid parameters in the ECW model are EA =8.66 and CA = 3.68.
These adducts, e.g. the complex with the tertiary amine DABCO, are safer to handle than TMA itself.[12]
The NASA ATREX mission (Anomalous Transport Rocket Experiment) employed the white smoke that TMA forms on air contact to study the high altitude jet stream.
Synthetic reagent
TMA is a source of methyl nucleophiles, akin to methyl lithium, but less reactive. It reacts with ketones to give, after a hydrolytic workup, tertiary alcohols.
Safety
Trimethylaluminium is pyrophoric, reacting violently with air and water.
References
- 1 2 3 4 5 6 Sigma-Aldrich Co., Trimethylaluminum. Retrieved on 2014-05-05.
- 1 2 3 4 5 "Trimethyl aluminum".
- ↑ Krause, Michael J.; Orlandi, Frank; Saurage, Alfred T.; Zietz, Joseph R. (2000). "Aluminum Compounds, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_543. ISBN 978-3527306732.
- ↑ C. Elschenbroich (2006). Organometallics. VCH. ISBN 978-3-527-29390-2.
- ↑ Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 0-12-352651-5.
- ↑ Vass, Gábor; Tarczay, György; Magyarfalvi, Gábor; Bödi, András; Szepes, László (2002). "HeI Photoelectron Spectroscopy of Trialkylaluminium and Dialkylaluminium Hydride Compounds and Their Oligomers". Organometallics. 21 (13): 2751–2757. doi:10.1021/om010994h.
- ↑ Lipton, Michael F.; Basha, Anwer; Weinreb, Steven M. (1979). "Conversion of Esters to Amides with Dimethylaluminium Amides: N,N-Dimethylcyclohexanecarboxamide". Organic Syntheses. 59: 49. doi:10.15227/orgsyn.059.0049.
- ↑ Gaines, D. F.; Borlin, Jorjan; Fody, E. P. (1974). "Trimethylgallium". Inorganic Syntheses. Inorganic Syntheses. Vol. 15. pp. 203–207. doi:10.1002/9780470132463.ch45. ISBN 9780470132463.
- ↑ Pine, S. H.; Kim, V.; Lee, V. (1990). "Enol ethers by methylenation of esters: 1-Phenoxy-1-phenylethene and 3,4-dihydro-2-methylene-2H-1-benzopyran". Org. Synth. 69: 72. doi:10.15227/orgsyn.069.0072.
- ↑ Negishi, E.; Matsushita, H. (1984). "Palladium-Catalyzed Synthesis of 1,4-Dienes by Allylation of Alkenyalane: α-Farnesene [1,3,6,10-Dodecatetraene, 3,7,11-trimethyl-]". Organic Syntheses. 62: 31. doi:10.15227/orgsyn.062.0031.
- ↑ Henrickson, C. H.; Duffy, D.; Eyman, D. P. (1968). "Lewis acidity of Alanes. Interactions of Trimethylalane with Amines, Ethers, and Phosphines". Inorganic Chemistry. 7 (6): 1047–1051. doi:10.1021/ic50064a001.
- ↑ Vinogradov, Andrej; Woodward, S. (2010). "Palladium-Catalyzed Cross-Coupling Using an Air-Stable Trimethylaluminium Source. Preparation of Ethyl 4-Methylbenzoate". Organic Syntheses. 87: 104. doi:10.15227/orgsyn.087.0104.
