 | |
| Names | |
|---|---|
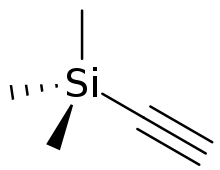
| Preferred IUPAC name
Ethynyltri(methyl)silane | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | TMSA |
| ChemSpider | |
| ECHA InfoCard | 100.012.655 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H10Si | |
| Molar mass | 98.220 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.69 g/mL |
| Boiling point | 53 °C (127 °F; 326 K) |
| Hazards | |
| GHS labelling: | |
   | |
| Danger | |
| H225, H315, H318, H319, H335 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P312, P321, P332+P313, P337+P313, P362, P370+P378, P403+P233, P403+P235, P405, P501 | |
| Safety data sheet (SDS) | External MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Trimethylsilylacetylene is the organosilicon compound with the formula (CH3)3SiC2H. A colorless liquid, "tms acetylene", as it is also called, is used as a source of "HC2−" in organic synthesis.
Use
Trimethylsilylacetylene is used in Sonogashira couplings as the equivalent of acetylene.[1] Using this protected alkyne, as opposed to acetylene itself, prevents further coupling reactions. The trimethylsilyl group can then be cleaved off with TBAF or DBU, either separately or as part of a one-pot Sonogashira reaction to form phenylacetylene derivatives.[2] A less expensive alternative reagent is 2-methylbut-3-yn-2-ol, which after alkynylation is deprotected with base.
Trimethylsilylacetylene is commercially available. It may also be prepared in a manner similar to other silyl compounds: deprotonation of acetylene with a Grignard reagent, followed by reaction with trimethylsilyl chloride.[3]
Trimethylsilylacetylene is a precursor to 1,4-bis(trimethylsilyl)buta-1,3-diyne, a protected form of 1,3-butadiyne.[4]
History
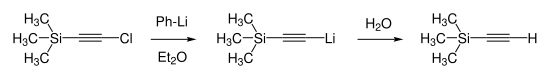
Trimethylsilylacetylene was first synthesized in 1959 by Heinz Günter Viehe. He reduced chloro(trimethylsilyl)acetylene by reaction with phenyllithium in diethyl ether and proceeded with subsequent hydrolysis.[5]
References
- ↑ Mio, Matthew J.; Kopel, Lucas C.; Braun, Julia B.; Gadzikwa, Tendai L.; Hull, Kami L.; Brisbois, Ronald G.; Markworth, Christopher J.; Grieco, Paul A. (2002). "One-Pot Synthesis of Symmetrical and Unsymmetrical Bisarylethynes by a Modification of the Sonogashira Coupling Reaction". Organic Letters. 4 (19): 3199–3202. doi:10.1021/ol026266n. PMID 12227748.
- ↑ Godson C. Nwokogu; Saskia Zemolka; Florian Dehme (2007). "Trimethylsilylacetylene". EROS. doi:10.1002/047084289X.rt288.pub2. ISBN 978-0-471-93623-7.
- ↑ Andrew B. Holmes, Chris N. Sporikou (1987). "Trimethylsilylacetylene". Organic Syntheses: 61. doi:10.15227/orgsyn.065.0061.
- ↑ Graham E. Jones, David A. Kendrick, and Andrew B. Holmes (1987). "1,4-Bis(trimethylsilyl)buta-1,3-diyne". Organic Syntheses. 65: 52. doi:10.15227/orgsyn.065.0052.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ H. G. Viehe (1959), "Heterosubstituierte Acetylene, III. Nucleophile Substitutionen und Halogen-Metall-Austauschreaktionen an Dreifachbindungen", Chemische Berichte (in German), vol. 92, no. 12, pp. 3064–3075, doi:10.1002/cber.19590921209