 | |
| Names | |
|---|---|
| Other names
Sodium trimetaphosphate | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.029.171 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Na3P3O9 | |
| Molar mass | 305.885 g/mol |
| Appearance | colorless or white crystals |
| Density | 2.49 g/cm3 (anhydrous) 1.786 g/cm3 (hexahydrate) |
| Melting point | 53 °C (127 °F; 326 K) (hexahydrate, decomposes to anyhdrous) |
| 22 g/100 mL | |
| Solubility | insoluble in alcohol |
Refractive index (nD) |
1.433 (hexahydrate) |
| Structure | |
| triclinic (hexahydrate) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
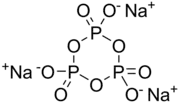
Sodium trimetaphosphate (also STMP), with formula Na3P3O9, is one of the metaphosphates of sodium. It has the formula Na3P3O9 but the hexahydrate Na3P3O9·(H2O)6 is also well known. It is the sodium salt of trimetaphosphoric acid. It is a colourless solid that finds specialised applications in food and construction industries.[2]
Although drawn with a particular resonance structure, the trianion has high symmetry.[3]
3(CollCode345).png.webp)
Synthesis and reactions
Trisodium trimetaphosphate is produced industrially by heating sodium dihydrogen phosphate to 550 °C, a method first developed in 1955:[5]
- 3 NaH2PO4 → Na3P3O9 + 3 H2O
The trimetaphosphate dissolves in water and is precipitated by the addition of sodium chloride (common ion effect), affording the hexahydrate.[6] STMP can also prepared by heating samples of sodium polyphosphate,[2] or by a thermal reaction of orthophosphoric acid and sodium chloride at 600°C.[7][8]
- 3 NaH3PO4 + 3 NaCl → Na3P3O9 + 3 H2O + 3 HCl
Hydrolysis of the ring leads to the acyclic sodium triphosphate:
- Na3P3O9 + H2O → H2Na3P3O10
The analogous reaction of the metatriphosphate anion involves ring-opening by amine nucleophiles.[9]
References
- ↑ Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, Florida: CRC Press. pp. 4–86. ISBN 0-8493-0594-2.
- 1 2 Klaus Schrödter; Gerhard Bettermann; Thomas Staffel; Friedrich Wahl; Thomas Klein; Thomas Hofmann (2008). "Phosphoric Acid and Phosphates". Ullmann's Encyclopedia of Industrial Chemistry. Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_465.pub3. ISBN 978-3527306732. S2CID 94458523.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 530. ISBN 978-0-08-037941-8.
- ↑ Tordjman, I.; Durif, A.; Guitel, J. C. (1976). "Structure Cristalline du Trimétaphosphate de Sodium Hexahydraté: Na3P3O9*6H2O". Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry. 32 (6): 1871–1874. doi:10.1107/S0567740876006560.
- ↑ Thilo, Erich; Grunze, Herbert (December 1955). "Zur Chemie der kondensierten Phosphate und Arsenate. XIII. Der Entwässerungsverlauf der Dihydrogenmonophosphate des Li, Na, K, und NH4". Zeitschrift für anorganische und allgemeine Chemie. 281 (5–6): 262–283. doi:10.1002/zaac.19552810504.
- ↑ Bell, R. N. (1950). "Sodium Metaphosphates". Inorganic Syntheses. Inorganic Syntheses. Vol. 3. pp. 103–106. doi:10.1002/9780470132340.ch26. ISBN 9780470132340.
- ↑ Minh, Doan Pham; Ramaroson, Jocelyn; Nzihou, Ange; Sharrock, Patrick; Depelsenaire, Guy (1 January 2012). "A New Route for the Synthesis of Alkali Polyphosphate from Economical Starting Materials: Preparation and Characterization of Sodium Cyclotriphosphate" (PDF). Phosphorus, Sulfur, and Silicon and the Related Elements. 187 (1): 112–120. doi:10.1080/10426507.2011.590950. S2CID 98164275.
- ↑ Pham Minh, Doan; Ramaroson, Jocelyn; Nzihou, Ange; Sharrock, Patrick (14 March 2012). "One-Step Synthesis of Sodium Trimetaphosphate (Na 3 P 3 O 9 ) from Sodium Chloride and Orthophosphoric Acid" (PDF). Industrial & Engineering Chemistry Research. 51 (10): 3851–3854. doi:10.1021/ie201085b.
- ↑ Bezold, Dominik; Dürr, Tobias; Singh, Jyoti; Jessen, Henning J. (2020). "Cyclotriphosphate: A Brief History, Recent Developments, and Perspectives in Synthesis". Chemistry – A European Journal. 26 (11): 2298–2308. doi:10.1002/chem.201904433. PMC 7065162. PMID 31637774.