The Tscherniak-Einhorn reaction is an organic chemistry name reaction initiated in 1901 by Joseph Tscherniak. It involves the condensation of N- hydroxymethylphthalimide with varied aromatic compounds. This process was later expanded upon in 1905 by Alfred Einhorn to include the condensation of N- hydroxymethylchloroacetamide and benzoic or cinnamic acid.[1][2]
Overview of reaction
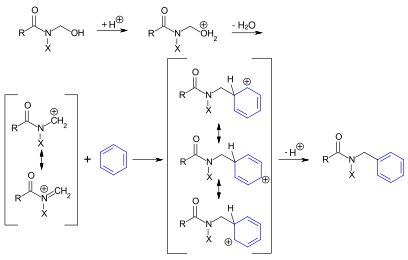
This reaction entails an acid-catalyzed Electrophilic aromatic substitution amidoalkylation, utilizing an N-hydroxymethylamide or N-hydroxymethylimide, which are also known as the Tscherniak-Einhorn reagents. The reaction is catalyzed by potent acids such as 85-100% sulfuric acid, p-toluenesulfonic acid, methanesulfonic acid, or trifluoroacetic acid.[2]
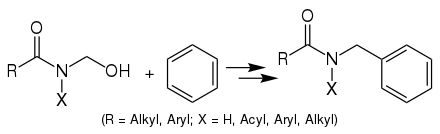
N-hydroxymethylamides may be prepared by the condensation of corresponding amides with an aqueous formaldehyde solution in dioxane, in the presence of sodium hydroxide.[3]
Reaction mechanism
In the first step, the N-hydroxymethylamide is subject to acid protonation. Post water elimination, a mesomerically stabilized cation forms. This reacts with the aromatic compound in line with an electrophilic aromatic substitution process.[2]
Applications
The Tscherniak-Einhorn reaction is used to synthesize some alkaloid derivatives.[2]
Literature
- Harold E. Zaugg, Ann M. Kotre, Jean E. Fraser (January 1969), "Tscherniac-Einhorn reaction. I. Equilibria in solutions of N-(hydroxymethyl)phthalimide in strong sulfuric acid", The Journal of Organic Chemistry, vol. 34, no. 1, pp. 11–13, doi:10.1021/jo00838a004
{{citation}}: CS1 maint: multiple names: authors list (link) - Harold E. Zaugg, Robert W. DeNet, Jean E. Fraser, Ann M. Kotre (January 1969), "Tscherniac-Einhorn reaction. II. Kinetics and mechanism", The Journal of Organic Chemistry, vol. 34, no. 1, pp. 14–18, doi:10.1021/jo00838a005
{{citation}}: CS1 maint: multiple names: authors list (link)
References
- ↑ Alfred Einhorn, Eduard Bischkopff, Bruno Szelinski, Gustav Schupp, Eduard Spröngerts, Carl Ladisch, Theodor Mauermayer (1905), "Ueber die N-Methylolverbindungen der Säureamide [Erste Abhandlung.]", Justus Liebig's Annalen der Chemie, vol. 343, no. 2–3, p. 207, doi:10.1002/jlac.19053430207
{{citation}}: CS1 maint: multiple names: authors list (link) - 1 2 3 4 "Tscherniac-Einhorn Reaction", Comprehensive Organic Name Reactions and Reagents, Wiley, pp. 2807–2811, 2010, doi:10.1002/9780470638859.conrr629, ISBN 978-0-470-63885-9
- ↑ "Tscherniac-Einhorn-Reaktion". illumina-chemie.de. 2013-05-11. Retrieved 2019-02-23.