 | |
| Names | |
|---|---|
| IUPAC names
Tungsten(V) fluoride Tungsten pentafluoride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| F5W | |
| Molar mass | 278.83 g·mol−1 |
| Appearance | yellow solid |
| Density | 5.01 g/cm3 |
| Melting point | 66 °C (151 °F; 339 K) |
| Boiling point | 215.6 °C (420.1 °F; 488.8 K) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
oxidizer, hydrolyzes to release HF |
| Flash point | Non-flammable |
| Related compounds | |
Related compounds |
TaCl5 NbCl5 MoF5 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
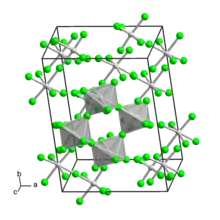
Tungsten(V) fluoride is an inorganic compound with the formula WF5. It is a hygroscopic yellow solid. Like most pentafluorides, it adopts a tetrameric structure, consisting of [WF5]4 molecules. In this way, each W center achieves octahedral coordination.[1]
Production
Tungsten(V) fluoride is produced by the reaction of tungsten and tungsten hexafluoride:[2]
- W + 5 WF6 → 6 WF5
At room temperature, it disproportionates to the tetra- and hexafluoride:
- 2 WF5 → WF4 + WF6
References
- ↑ Edwards, A. J. (1969). "Crystal Structure of tungsten pentafluoride". J. Chem. Soc. A: 909. doi:10.1039/J19690000909.
- ↑ Schröder, Johann; Grewe, Franz J. (1970). "Darstellung und Eigenschaften von Wolframpentafluorid". Chemische Berichte. 103 (5): 1536–46. doi:10.1002/cber.19701030524.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.